Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo
- PMID: 33488738
- PMCID: PMC7801080
- DOI: 10.1155/2021/6613029
Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo
Abstract
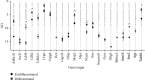
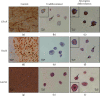
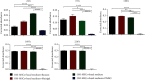
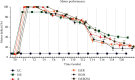
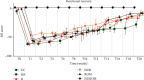
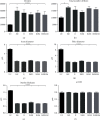
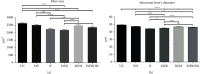
Peripheral nerve injury remains a clinical challenge with severe physiological and functional consequences. Despite the existence of multiple possible therapeutic approaches, until now, there is no consensus regarding the advantages of each option or the best methodology in promoting nerve regeneration. Regenerative medicine is a promise to overcome this medical limitation, and in this work, chitosan nerve guide conduits and olfactory mucosa mesenchymal stem/stromal cells were applied in different therapeutic combinations to promote regeneration in sciatic nerves after neurotmesis injury. Over 20 weeks, the intervened animals were subjected to a regular functional assessment (determination of motor performance, nociception, and sciatic indexes), and after this period, they were evaluated kinematically and the sciatic nerves and cranial tibial muscles were evaluated stereologically and histomorphometrically, respectively. The results obtained allowed confirming the beneficial effects of using these therapeutic approaches. The use of chitosan NGCs and cells resulted in better motor performance, better sciatic indexes, and lower gait dysfunction after 20 weeks. The use of only NGGs demonstrated better nociceptive recoveries. The stereological evaluation of the sciatic nerve revealed identical values in the different parameters for all therapeutic groups. In the muscle histomorphometric evaluation, the groups treated with NGCs and cells showed results close to those of the group that received traditional sutures, the one with the best final values. The therapeutic combinations studied show promising outcomes and should be the target of new future works to overcome some irregularities found in the results and establish the combination of nerve guidance conduits and olfactory mucosa mesenchymal stem/stromal cells as viable options in the treatment of peripheral nerves after injury.
Copyright © 2021 Rui D. Alvites et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures









References
-
- Alvites R. D., Branquinho M. V., Caseiro A. R., et al. Biomaterials and Cellular Systems at the Forefront of Peripheral Nerve Regeneration. Peripheral Nerves-Injuries, Disorders and Treatment: Intech Open; 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources

