Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for treatment of recurrent or metastatic head and neck squamous cell carcinoma: a systematic review and network meta-analysis
- PMID: 33488783
- PMCID: PMC7768319
- DOI: 10.1177/1758835920983717
Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for treatment of recurrent or metastatic head and neck squamous cell carcinoma: a systematic review and network meta-analysis
Abstract
Background: Multiple therapies including immune-checkpoint inhibitors are emerging as effective treatment for patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSSC). However, the optimal first-line and second-line treatments remains controversial.
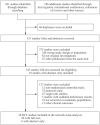
Methods: We systematically searched databases and conducted a systematic review of phase II/III randomized controlled trials (RCTs) that compared two or more treatments for R/M HNSSC. Progression-free survival (PFS), overall survival (OS) and adverse events (AEs) ⩾3 with hazard ratios (HRs) were extracted and synthesized based on a frequentist network meta-analysis.
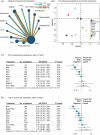
Results: Twenty-six trials involving 8908 patients were included. Of first-line treatments, pembrolizumab plus cisplatin plus 5-fluorouracil is associated with significantly improved OS (P-score = 0.91) and TPEx ranked first for prolonging PFS (0.91). EXTREME plus docetaxel (0.18) ranked lowest for AEs ⩾3. Of second-line treatments, nivolumab was the highest-ranked treatment for prolonging OS (0.95), while buparlisib plus paclitaxel was the highest-ranked treatment for PFS (0.94). Subgroup analyses suggested that nivolumab was significantly associated with improvement of OS in patients with high PD-L1 expression (HR 0.55, 0.43-0.70), whereas its OS benefit is similar with conventional chemotherapy for those with low PD-L1 expression. Buparlisib plus paclitaxel showed the best OS benefit in subgroups of patients with HPV-negative status, and with oral cavity or larynx as primary tumor sites.
Conclusions: Pembrolizumab plus cisplatin plus 5-fluorouracil is likely to be the best first-line treatment when OS is a priority. Otherwise, TPEx should be the optimal first-line option due to its superior PFS prolongation efficacy, best safety profile, and similar OS benefit with pembrolizumab plus cisplatin plus 5-fluorouracil. Nivolumab appears to be the best second-line option with best OS prolongation efficacy and outstanding safety profile in the overall population. Future RCTs with meticulous grouping of patients and detailed reporting are urgently needed for individualized treatment.
Keywords: chemotherapy; immune-checkpoint inhibitor; network meta-analysis; recurrent or metastatic head and neck carcinoma.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. - PubMed
-
- Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology 2016; 91: 13–23. - PubMed
-
- Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol 2015; 33: 3305–3313. - PubMed
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019; 394: 1915–1928. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

