Exosomes Derived from Human Urine-Derived Stem Cells Inhibit Intervertebral Disc Degeneration by Ameliorating Endoplasmic Reticulum Stress
- PMID: 33488928
- PMCID: PMC7787770
- DOI: 10.1155/2020/6697577
Exosomes Derived from Human Urine-Derived Stem Cells Inhibit Intervertebral Disc Degeneration by Ameliorating Endoplasmic Reticulum Stress
Abstract
Objective: This study is aimed at determining the effects of human urine-derived stem cell-derived exosomes (USCs-exos) on pressure-induced nucleus pulposus cell (NPC) apoptosis and intervertebral disc degeneration (IDD) and on the ERK and AKT signaling pathways.
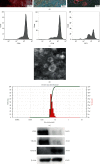
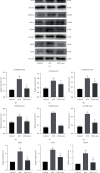
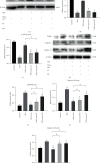
Methods: The NPCs were obtained from patients with herniated lumbar discs. Western blot analysis (WB) and quantitative real-time polymerase chain reaction (qRT-PCR) were used to determine endoplasmic reticulum (ER) stress levels of NPCs under stress. Human USCs were identified using an inverted microscope, three-line differentiation experiments, and flow cytometry. A transmission microscope, nanoparticle size analysis, and WB procedures were used to identify the extracted exosomes and observe NPC uptake. A control group, a 48 h group, and a USCs-exos group were established. The control group was untreated, and the 48 h group was pressure-trained for 48 h, while the USCs-exos group was pressure-trained for 48 h and treated with USCs-exos. WB, qRT-PCR, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis were used to determine the ER stress levels in stress conditions and after exosomal treatment. The AKT and ERK pathways were partially detected. Magnetic Resonance Imaging (MRI) and computed tomography (CT) were used to evaluate cell degeneration while exosomal effects on the intervertebral disc (IVD) tissue were determined by hematoxylin and eosin (HE) staining, Safranin O-fast green staining, immunohistochemical staining (IHC), nuclear magnetic resonance (NMR), spectrometric detection, and total correlation spectroscopy (TOCSY). IVD metabolites were also identified and quantified.
Results: After pressure culture, ER stress markers (GRP78 and C/EBP homologous protein (CHOP)) in the NPCs were significantly elevated with time (p < 0.05). Human USCs are short and spindle-shaped. They can successfully undergo osteogenic, adipogenic, and chondrogenic differentiation. In this study, these stem cells were found to be positive for CD29, CD44, and CD73. The exosomes were centrally located with a diameter of 50-100 nm. CD63 and Tsg101 were highly expressed while the expression of Calnexin was suppressed. The exosomes can be ingested by NPCs. USCs-exos significantly improved ER stress responses and inhibited excessive activation of the unfolded protein response (UPR) as well as cell apoptosis and disc degeneration through the AKT and ERK signaling pathways (p < 0.05).
Conclusion: Through the AKT and ERK signaling pathways, USCs-exos significantly inhibit ER stress-induced cell apoptosis and IDD under pressure conditions. It is, therefore, a viable therapeutic strategy.
Copyright © 2020 HongFei Xiang et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Macfarlane G. J., Thomas E., Croft P. R., Papageorgiou A. C., Jayson M. I. V., Silman A. J. Predictors of early improvement in low back pain amongst consulters to general practice: the influence of pre-morbid and episode-related factors. Pain. 1999;80(1):113–119. doi: 10.1016/S0304-3959(98)00209-7. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

