A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways
- PMID: 33488943
- PMCID: PMC7803406
- DOI: 10.1155/2021/7689045
A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways
Abstract
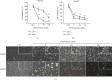
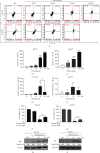
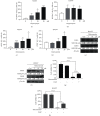
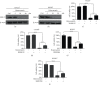
Hepatocellular carcinoma (HCC) is a leading cause of death, resulting in over 700 thousand deaths annually worldwide. Chemotherapy is the primary therapeutic strategy for patients with late-stage HCC. Heteronemin is a marine natural product isolated from Hippospongia sp. that has been found to protect against carcinogenesis in cholangiocarcinoma, prostate cancer, and acute myeloid leukemia. In this study, heteronemin was found to inhibit the proliferation of the HCC cell lines HA22T and HA59T and induce apoptosis via the caspase pathway. Heteronemin treatment also induced the formation of reactive oxygen species (ROS), which are associated with heteronemin-induced cell death, and to trigger ROS removal by mitochondrial SOD2 rather than cytosolic SOD1. The mitogen-activated protein kinase (MAPK) signaling pathway was associated with ROS-induced cell death, and heteronemin downregulated the expression of ERK, a MAPK that is associated with cell proliferation. Inhibitors of JNK and p38, which are MAPKs associated with apoptosis, restored heteronemin-induced cell death. In addition, heteronemin treatment reduced the expression of GPX4, a protein that inhibits ferroptosis, which is a novel form of nonapoptotic programmed cell death. Ferroptosis inhibitor treatment also restored heteronemin-induced cell death. Thus, with appropriate structural modification, heteronemin can act as a potent therapeutic against HCC.
Copyright © 2021 Wen-Tsan Chang et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Miyamoto M., Takano M., Kuwahara M., et al. Efficacy of combination chemotherapy using irinotecan and nedaplatin for patients with recurrent and refractory endometrial carcinomas: preliminary analysis and literature review. Cancer Chemotherapy and Pharmacology. 2018;81(1):111–117. doi: 10.1007/s00280-017-3454-y. - DOI - PubMed
-
- Demetri G. D., von Mehren M., Jones R. L., et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. Journal of Clinical Oncology. 2016;34(8):786–793. doi: 10.1200/JCO.2015.62.4734. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

