Sweet and Blind Spots in E3 Ligase Ligand Space Revealed by a Thermophoresis-Based Assay
- PMID: 33488967
- PMCID: PMC7812675
- DOI: 10.1021/acsmedchemlett.0c00440
Sweet and Blind Spots in E3 Ligase Ligand Space Revealed by a Thermophoresis-Based Assay
Erratum in
-
Correction to "Sweet and Blind Spots in E3 Ligase Ligand Space Revealed by a Thermophoresis-Based Assay".ACS Med Chem Lett. 2021 Dec 28;13(1):148-149. doi: 10.1021/acsmedchemlett.1c00693. eCollection 2022 Jan 13. ACS Med Chem Lett. 2021. PMID: 35047112 Free PMC article.
Abstract
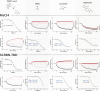
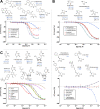
Repurposing E3 ubiquitin ligases for targeted protein degradation via customized molecular glues or proteolysis-targeting chimeras (PROTACs) is an increasingly important therapeutic modality. Currently, a major limitation in the design of suitable molecular glues and PROTACs is our fragmentary understanding of E3 ligases and their ligand space. We here describe a quantitative assay for the discovery and characterization of E3 ligase ligands that is based on the thermophoretic behavior of a custom reporter ligand. Thereby, it is orthogonal to commonly employed fluorescence-based assays and less affected by the optical properties of test compounds. It can be employed for the high-throughput screening of compound libraries for a given ligase but also for hit validation, which we demonstrate with the identification of unexpected well-binders and non-binders, yielding new insights into the ligand space of cereblon (CRBN).
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

