Synthesis and Metabolism of BTN3A1 Ligands: Studies on Modifications of the Allylic Alcohol
- PMID: 33488975
- PMCID: PMC7812676
- DOI: 10.1021/acsmedchemlett.0c00586
Synthesis and Metabolism of BTN3A1 Ligands: Studies on Modifications of the Allylic Alcohol
Abstract
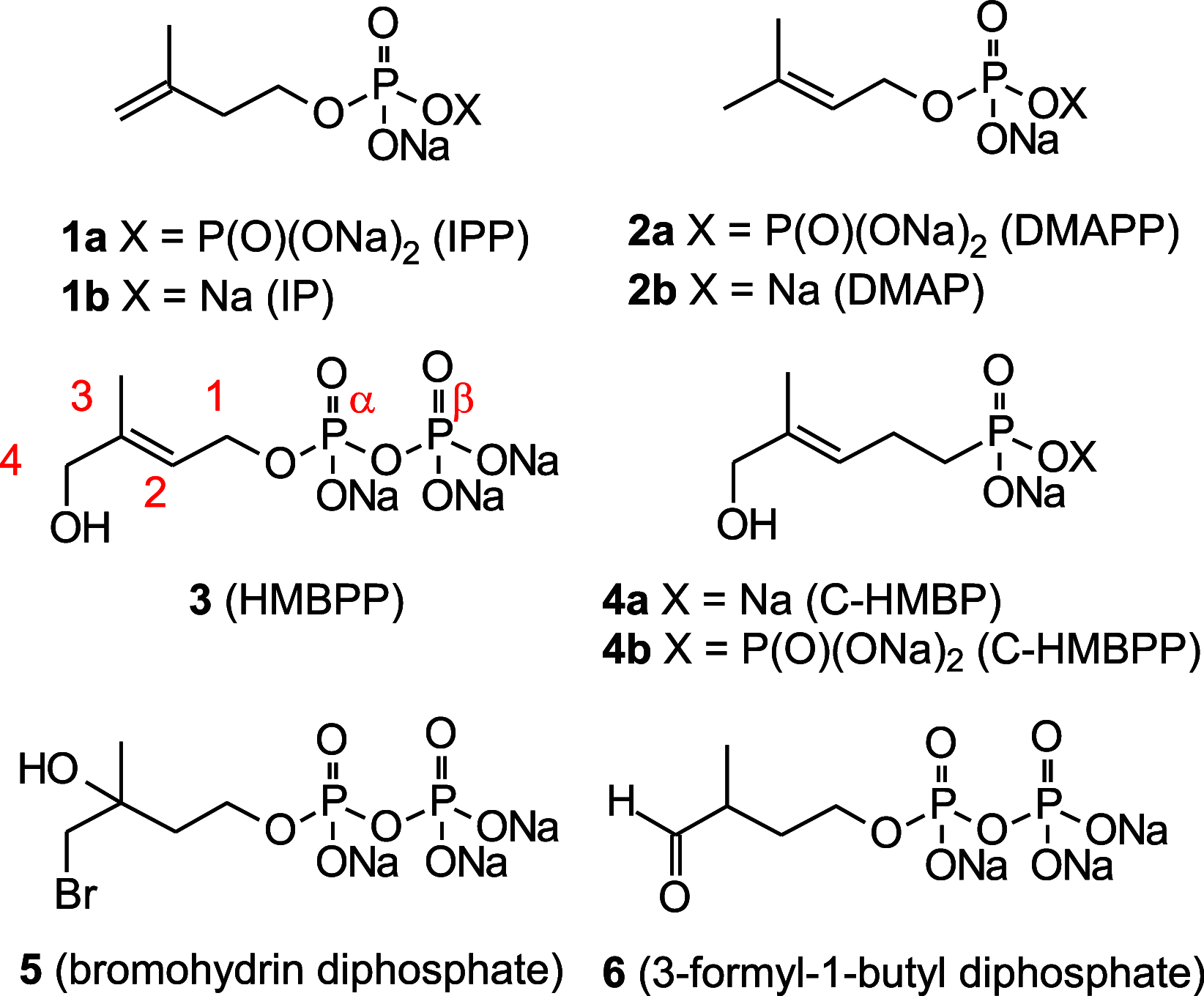
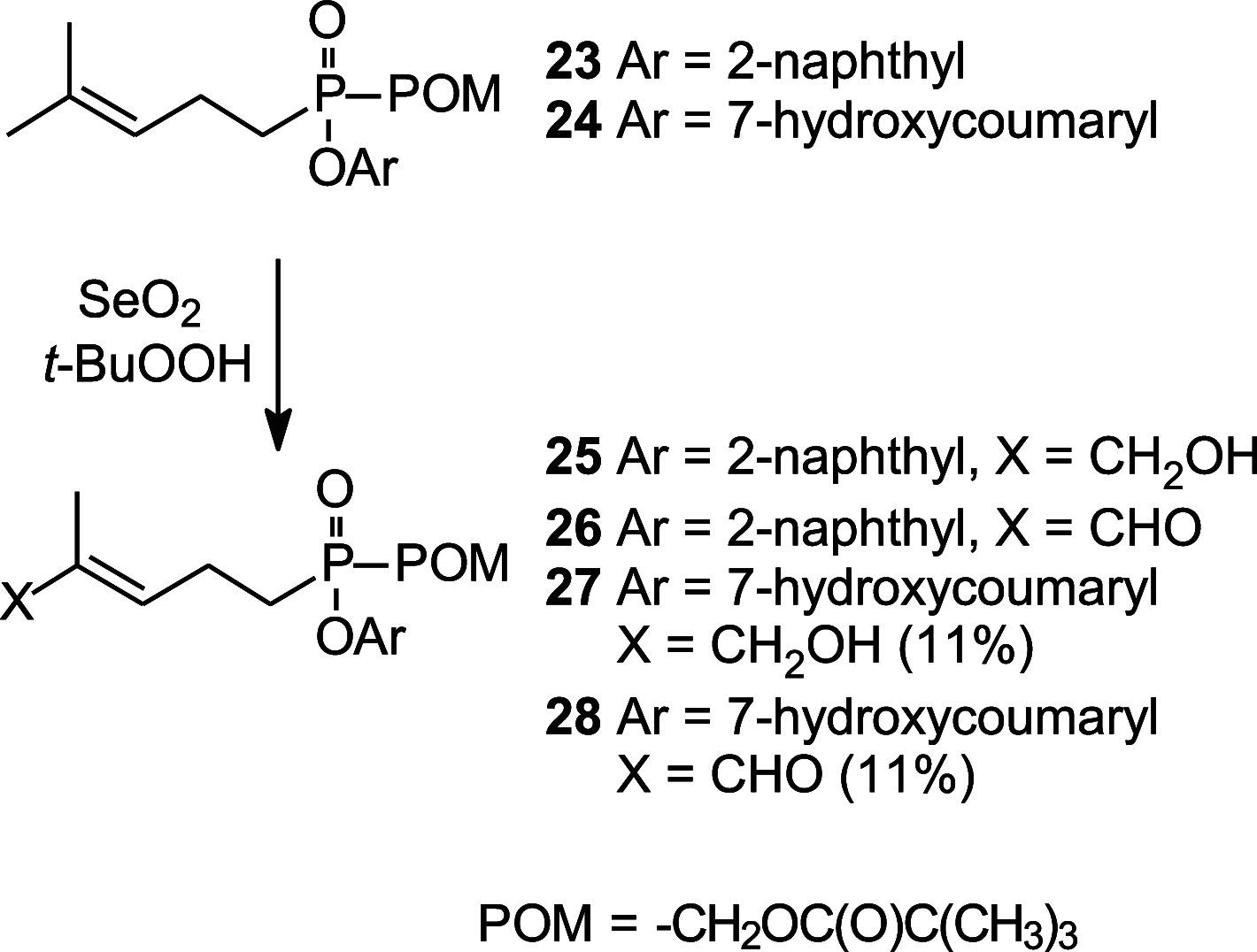
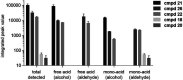
(E)-4-Hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) and its phosphonate analogs are potent phosphoantigens. HMBPP contains an (E)-allylic alcohol which interacts with the molecular target BTN3A1 giving an antigenic signal to activate Vγ9Vδ2 T cells. As probes of BTN3A1 function, we prepared prodrug derivatives of the HMBPP analog C-HMBP that lack the (E)-allylic alcohol or have modified it to an aldehyde or aldoxime and evaluated their biological activity. Removal of the alcohol completely abrogates phosphoantigenicity in these compounds while the aldoxime modification decreases potency relative to the (E)-allylic alcohol form. However, homoprenyl derivatives oxidized to an aldehyde stimulate Vγ9Vδ2 T cells at nanomolar concentrations. Selection of phosphonate protecting groups (i.e., prodrug forms) impacts the potency of phosphoantigen aldehydes, with mixed aryl acyloxyalkyl forms exhibiting superior activity relative to aryl amidate forms. The activity correlates with the cellular reduction of the aldehyde to the alcohol form. Thus, the functionality on this ligand framework can be altered concurrently with phosphonate protection to promote cellular transformation to highly potent phosphoantigens.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.J.W. and D.F.W. own shares in Terpenoid Therapeutics, Inc. The current work did not involve the company. The other authors have no financial conflicts of interest.
Figures




References
-
- Morita C. T.; Jin C.; Sarikonda G.; Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. 10.1111/j.1600-065X.2006.00479.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

