Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium
- PMID: 33489012
- PMCID: PMC7810129
- DOI: 10.1002/jev2.12044
Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium
Abstract
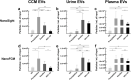
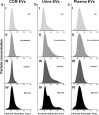
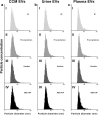
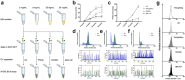
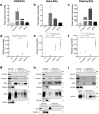
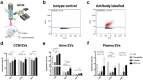
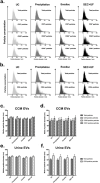
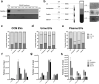
One of the challenges that restricts the evolving extracellular vesicle (EV) research field is the lack of a consensus method for EV separation. This may also explain the diversity of the experimental results, as co-separated soluble proteins and lipoproteins may impede the interpretation of experimental findings. In this study, we comprehensively evaluated the EV yields and sample purities of three most popular EV separation methods, ultracentrifugation, precipitation and size exclusion chromatography combined with ultrafiltration, along with a microfluidic tangential flow filtration device, Exodisc, in three commonly used biological samples, cell culture medium, human urine and plasma. Single EV phenotyping and density-gradient ultracentrifugation were used to understand the proportion of true EVs in particle separations. Our findings suggest Exodisc has the best EV yield though it may co-separate contaminants when the non-EV particle levels are high in input materials. We found no 100% pure EV preparations due to the overlap of their size and density with many non-EV particles in biofluids. Precipitation has the lowest sample purity, regardless of sample type. The purities of the other techniques may vary in different sample types and are largely dependent on their working principles and the intrinsic composition of the input sample. Researchers should choose the proper separation method according to the sample type, downstream analysis and their working scenarios.
Keywords: EV yield; methods comparison; sample purity; single particle phenotyping; small extracellular vesicle separation.
© 2020 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
UNIST has filed patents on Exodisc and YKC is named as an inventor, which are licensed to LabSpinner, Inc.
Figures

References
-
- Benedikter, B. J. , Bouwman, F. G. , Vajen, T. , Heinzmann, A. C. , Grauls, G. , Mariman, E. C. , … Rohde, G. G. (2017). Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Scientific Reports, 7(1), 1–3. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

