N-acetylcysteine-loaded electrospun mats improve wound healing in mice and human fibroblast proliferation in vitro: a potential application of nanotechnology in wound care
- PMID: 33489034
- PMCID: PMC7811817
- DOI: 10.22038/ijbms.2020.41550.11078
N-acetylcysteine-loaded electrospun mats improve wound healing in mice and human fibroblast proliferation in vitro: a potential application of nanotechnology in wound care
Abstract
Objectives: N-acetylcysteine (NAC) has gained attention recently in dermatology as a unique anti-oxidant. In light of progress in nanotechnological methods, it was hypothesized that loading NAC onto nanofibers would positively affect skin wound healing. The objective of this study was to fabricate NAC-loaded electrospun mats and test their effect on wound healing in vivo and in vitro.
Materials and methods: Polyvinyl alcohol (PVA)-based mats loaded with NAC at three concentrations were electrospun and characterized in terms of physicochemical properties and drug release profile. Human fibroblast cells (in vitro) and mouse full-thickness skin wounds (in vivo) were treated with mats for 5 and 14 days, respectively. Wound area, tissue histopathology, fibroblast proliferation and cellular oxidative state were evaluated.
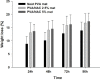
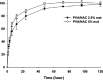
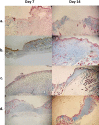
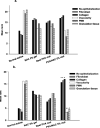
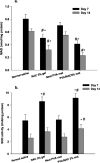
Results: Mats containing 5% PVA/NAC showed thinner fibers with suitable physicochemical properties and a sustained drug release profile. PVA/NAC (5%) mats enhanced fibroblast proliferation and attachment in vitro. The mats resulted in significant wound closure with high levels of re-epithelialization and collagen fiber synthesis on day 14 post-surgery in vivo. The mats also reduced granulation tissue and edematous stroma to a higher extent. These findings were accompanied by a significant decrease in tissue lipid peroxidation and higher superoxide dismutase activity, which may explain how NAC improved wound healing.
Conclusion: We propose an NAC-loaded nanofibrous mat that takes the advantage of a porous nanoscaffold structure to release NAC in a sustained manner. This mat may be a promising candidate for further clinical evaluation.
Keywords: Electrospun nanofiber; In vitro; Mouse; N-acetylcysteine (NAC); Oxidative stress; Wound.
Figures





References
-
- Kumar Chellappan D, Yenese Y, Chian Wei C, Gupta G. Nanotechnology and diabetic wound healing: a review. Endocr Metab Immune Disord Drug Targets. 2017;17:87–95. - PubMed
-
- Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing–a review. J Mater Chem B. 2013;1:4531–4541. - PubMed
-
- Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94. - PubMed
-
- Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–171. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
