Remdesivir therapy in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials
- PMID: 33489115
- PMCID: PMC7806502
- DOI: 10.1016/j.amsu.2020.12.051
Remdesivir therapy in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials
Abstract
Purpose: To perform a systematic review and meta-analysis of randomized controlled trials that examined remdesivir treatment for COVID-19.
Materials and methods: A systematic literature search was performed using Pubmed, Embase, and ClinicalTrials.gov to identify studies published up to October 25, 2020 that examined COVID-19 treatment with remdesivir. A total of 3 randomized controlled trials that consisted of 1691 patients were included in the meta-analysis.
Results: The odds for mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO) following treatment was significantly lower in the remdesivir group compared to the control group (OR = 0.48 [95% CI: 0.34; 0.69], p < 0.001). The odds of early (at day 14/15; OR = 1.42 [95% CI: 1.16; 1.74], p < 0.001) and late (at day 28/29; OR = 1.44 [95% CI: 1.16; 1.79], p = 0.001) hospital discharge were significantly higher in the remdesivir group compared to the control group. There was no difference in the odds for mortality in patients treated with remdesivir (OR = 0.77 [95% CI: 0.56; 1.06], p = 0.108).
Conclusions: Remdesivir attenuates disease progression, leading to lower odds of MV/ECMO and greater odds of hospital discharge for COVID-19 patients. However, remdesivir does not affect odds of mortality.
Keywords: Antiviral agents; Coronavirus; SARS virus; Therapeutic uses.
© 2020 Published by Elsevier Ltd on behalf of IJS Publishing Group Ltd.
Conflict of interest statement
The authors declare no interests with the subject of this manuscript. J.M.P. is employed by Nested Knowledge, Superior Medical Experts, and Marblehead Medical. K.M.K. works for and holds equity in Nested Knowledge, Superior Medical Experts, and Marblehead Medical. A.R.D. and K.W.E. are employed by Superior Medical Experts.
Figures



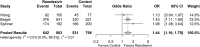

References
-
- Coronavirus disease COVID-19 Weekly epidemiological update and weekly operational update. 2020. https://www.who.int/publications/m/item/weekly-operational-update-on-cov... 7 December 2020.
-
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Northwell the, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. JAMA; 2020. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. - PMC - PubMed
-
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., Li J.F., Li C.C., Ou L.M., Cheng B., Xiong S., Ni Z.Y., Xiang J., Hu Y., Liu L., Shan H., Lei C.L., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Cheng L.L., Ye F., Li S.Y., Zheng J.P., Zhang N.F., Zhong N.S., He J.X. C. China medical treatment expert group for, comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5) - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

