Plasmablastic Lymphoma Associated with Adjacent Mature Plasma Cell Population Exhibiting Opposite Light Chain Restriction
- PMID: 33489398
- PMCID: PMC7803171
- DOI: 10.1155/2020/8875547
Plasmablastic Lymphoma Associated with Adjacent Mature Plasma Cell Population Exhibiting Opposite Light Chain Restriction
Abstract
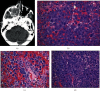
Plasmablastic lymphoma (PBL) is an aggressive high-grade B cell lymphoma, considered a variant of diffuse large B cell lymphoma with approximately 75% mortality within 6-7 months. We describe an unusual case of PBL arising as a maxillary mass in an HIV-negative, nontransplanted 78-year-old female. Histologic examination revealed a diffuse infiltrate of anaplastic appearing cells exhibiting plasmablastic morphology with an adjacent contiguous infiltrate of mature appearing plasma cells. The PBL and mature plasma cell components both demonstrated an immunophenotype of CD20(-), CD38(+), and CD138(+). The two populations differed by the PBL featuring a high proliferation rate by Ki-67 (~95%) with coexpression of both c-MYC and EBV, while the mature plasma cell component featured a low proliferation rate by Ki-67 (~5%) without coexpression of c-MYC or EBV. Kappa/lambda staining demonstrated lambda light chain restriction involving the PBL, while the mature plasma cell infiltrate revealed kappa light chain restriction. Our findings describe the rare association of PBL with a synchronous distinct population of mature plasma cells exhibiting opposite light chain restriction.
Copyright © 2020 Karina Furlan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Montes-Moreno S., Gonzalez-Medina A. R., Rodriguez-Pinilla S. M., et al. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010;95(8):1342–1349. doi: 10.3324/haematol.2009.016113. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

