Volumetric Regression in Brain Metastases After Stereotactic Radiotherapy: Time Course, Predictors, and Significance
- PMID: 33489888
- PMCID: PMC7820888
- DOI: 10.3389/fonc.2020.590980
Volumetric Regression in Brain Metastases After Stereotactic Radiotherapy: Time Course, Predictors, and Significance
Abstract
Background: There is insufficient understanding of the natural course of volumetric regression in brain metastases after stereotactic radiotherapy (SRT) and optimal volumetric criteria for the assessment of response and progression in radiotherapy clinical trials for brain metastases are currently unknown.
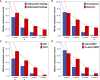
Methods: Volumetric analysis via whole-tumor segmentation in contrast-enhanced 1 mm³-isotropic T1-Mprage sequences before SRT and during follow-up. A total of 3,145 MRI studies of 419 brain metastases from 189 patients were segmented. Progression was defined using a volumetric extension of the RANO-BM criteria. A subset of 205 metastases without progression/radionecrosis during their entire follow-up of at least 3 months was used to study the natural course of volumetric regression after SRT. Predictors for volumetric regression were investigated. A second subset of 179 metastases was used to investigate the prognostic significance of volumetric response at 3 months (defined as ≥20% and ≥65% volume reduction, respectively) for subsequent local control.
Results: Median relative metastasis volume post-SRT was 66.9% at 6 weeks, 38.6% at 3 months, 17.7% at 6 months, 2.7% at 12 months and 0.0% at 24 months. Radioresistant histology and FSRT vs. SRS were associated with reduced tumor regression for all time points. In multivariate linear regression, radiosensitive histology (p=0.006) was the only significant predictor for metastasis regression at 3 months. Volumetric regression ≥20% at 3 months post-SRT was the only significant prognostic factor for subsequent control in multivariate analysis (HR 0.63, p=0.023), whereas regression ≥65% was no significant predictor.
Conclusions: Volumetric regression post-SRT does not occur at a constant rate but is most pronounced in the first 6 weeks to 3 months. Despite decreasing over time, volumetric regression continues beyond 6 months post-radiotherapy and may lead to complete resolution of controlled lesions by 24 months. Radioresistant histology is associated with slower regression. We found that a cutoff of ≥20% regression for the volumetric definition of response at 3 months post-SRT was predictive for subsequent control whereas the currently proposed definition of ≥65% was not. These results have implications for standardized volumetric criteria in future radiotherapy trials for brain metastases.
Keywords: MRI; brain metastases; longitudinal analysis; stereotactic radiosurgery; stereotactic radiotherapy; volumetric analysis; volumetric regression.
Copyright © 2021 Oft, Schmidt, Weissmann, Roesch, Mengling, Masitho, Bert, Lettmaier, Frey, Distel, Fietkau and Putz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res (2012) 32(11):4655–62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

