The Complex Interactions Between Rotavirus and the Gut Microbiota
- PMID: 33489932
- PMCID: PMC7819889
- DOI: 10.3389/fcimb.2020.586751
The Complex Interactions Between Rotavirus and the Gut Microbiota
Abstract
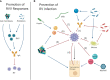
Human rotavirus (HRV) is the leading worldwide cause of acute diarrhea-related death in children under the age of five. RV infects the small intestine, an important site of colonization by the microbiota, and studies over the past decade have begun to reveal a complex set of interactions between RV and the gut microbiota. RV infection can temporarily alter the composition of the gut microbiota and probiotic administration alleviates some symptoms of infection in vivo, suggesting reciprocal effects between the virus and the gut microbiota. While development of effective RV vaccines has offered significant protection against RV-associated mortality, vaccine effectiveness in low-income countries has been limited, potentially due to regional differences in the gut microbiota. In this mini review, we briefly detail research findings to date related to HRV vaccine cohorts, studies of natural infection, explorations of RV-microbiota interactions in gnotobiotic pig models, and highlight various in vivo and in vitro models that could be used in future studies to better define how the microbiota may regulate RV infection and host antiviral immune responses.
Keywords: animal models; immunity; in vitro models; microbiota; rotavirus (human and animal); rotavirus vaccine.
Copyright © 2021 Kim, Hogarty, Harris and Baldridge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

