Hydroxyapatite Particle Density Regulates Osteoblastic Differentiation Through β-Catenin Translocation
- PMID: 33490047
- PMCID: PMC7820766
- DOI: 10.3389/fbioe.2020.591084
Hydroxyapatite Particle Density Regulates Osteoblastic Differentiation Through β-Catenin Translocation
Abstract
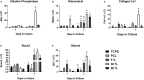
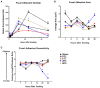
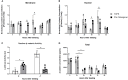
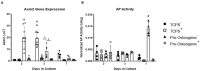
Substrate surface characteristics such as roughness, wettability and particle density are well-known contributors of a substrate's overall osteogenic potential. These characteristics are known to regulate cell mechanics as well as induce changes in cell stiffness, cell adhesions, and cytoskeletal structure. Pro-osteogenic particles, such as hydroxyapatite, are often incorporated into a substrate to enhance the substrates osteogenic potential. However, it is unknown which substrate characteristic is the key regulator of osteogenesis. This is partly due to the lack of understanding of how these substrate surface characteristics are transduced by cells. In this study substrates composed of polycaprolactone (PCL) and carbonated hydroxyapatite particles (HAp) were synthesized. HAp concentration was varied, and a range of surface characteristics created. The effect of each substrate characteristic on osteoblastic differentiation was then examined. We found that, of the characteristics examined, only HAp density, and indeed a specific density (85 particles/cm2), significantly increased osteoblastic differentiation. Further, an increase in focal adhesion maturation and turnover was observed in cells cultured on this substrate. Moreover, β-catenin translocation from the membrane bound cell fraction to the nucleus was more rapid in cells on the 85 particle/cm2 substrate compared to cells on tissue culture polystyrene. Together, these data suggest that particle density is one pivotal factor in determining a substrates overall osteogenic potential. Additionally, the observed increase in osteoblastic differentiation is a at least partly the result of β-catenin translocation and transcriptional activity suggesting a β-catenin mediated mechanism by which substrate surface characteristics are transduced.
Keywords: beta catenin; focal adhesion; mechanotransduction; surface topography; translocation.
Copyright © 2021 Juhl, Merife, Zhang, Lemmon and Donahue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bertocchi C., Ravasio A., Ong H. T., Toyama Y., Kanchanawong P. (2019). Mechanical roles of vinculin/β-catenin interaction in adherens junction. bioRxiv 770–735. 10.1101/770735 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

