Structural Roles for the Juxtamembrane Linker Region and Transmembrane Region of Synaptobrevin 2 in Membrane Fusion
- PMID: 33490074
- PMCID: PMC7815645
- DOI: 10.3389/fcell.2020.609708
Structural Roles for the Juxtamembrane Linker Region and Transmembrane Region of Synaptobrevin 2 in Membrane Fusion
Abstract
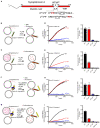
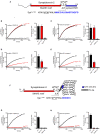
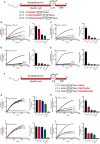
Formation of the trans-SNARE complex is believed to generate a force transfer to the membranes to promote membrane fusion, but the underlying mechanism remains elusive. In this study, we show that helix-breaking and/or length-increasing insertions in the juxtamembrane linker region of synaptobrevin-2 exert diverse effects on liposome fusion, in a manner dependent on the insertion position relative to the two conserved tryptophan residues (W89/W90). Helical extension of synaptobrevin-2 to W89/W90 is a prerequisite for initiating membrane merger. The transmembrane region of synaptobrevin-2 enables proper localization of W89/W90 at the membrane interface to gate force transfer. Besides, our data indicate that the SNARE regulatory components Munc18-1 and Munc13-1 impose liposome fusion strong demand on tight coupling between the SNARE motif and the transmembrane region of synaptobrevin-2.
Keywords: Munc13; Munc18; SNARE complex assembly; membrane fusion; synaptobrevin-2.
Copyright © 2021 Hu, Zhu and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

