Tissue-Resident Macrophage Development and Function
- PMID: 33490082
- PMCID: PMC7820365
- DOI: 10.3389/fcell.2020.617879
Tissue-Resident Macrophage Development and Function
Abstract
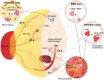
Tissue-resident macrophages have been associated with important and diverse biological processes such as native immunity, tissue homeostasis and angiogenesis during development and postnatally. Thus, it is critical to understand the origins and functions of tissue-resident macrophages, as well as mechanisms underlying their regulation. It is now well accepted that murine macrophages are produced during three consecutive waves of hematopoietic development. The first wave of macrophage formation takes place during primitive hematopoiesis, which occurs in the yolk sac, and gives rise to primitive erythroid, megakaryocyte and macrophage progenitors. These "primitive" macrophage progenitors ultimately give rise to microglia in the adult brain. The second wave, which also occurs in the yolk sac, generates multipotent erythro-myeloid progenitors (EMP), which give rise to tissue-resident macrophages. Tissue-resident macrophages derived from EMP reside in diverse niches of different tissues except the brain, and demonstrate tissue-specific functions therein. The third wave of macrophages derives from hematopoietic stem cells (HSC) that are formed in the aorta-gonad-mesonephros (AGM) region of the embryo and migrate to, and colonize, the fetal liver. These HSC-derived macrophages are a long-lived pool that will last throughout adulthood. In this review, we discuss the developmental origins of tissue-resident macrophages, their molecular regulation in specific tissues, and their impact on embryonic development and postnatal homeostasis.
Keywords: definitive hematopoiesis; erythro-myeloid progenitors; hemogenic endothelial cells; primitive hematopoiesis; tissue-resident macrophages.
Copyright © 2021 Wu and Hirschi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

