Phenotypically supervised single-cell sequencing parses within-cell-type heterogeneity
- PMID: 33490901
- PMCID: PMC7808958
- DOI: 10.1016/j.isci.2020.101991
Phenotypically supervised single-cell sequencing parses within-cell-type heterogeneity
Abstract
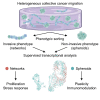
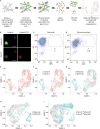
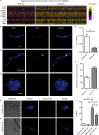
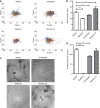
To better understand cellular communication driving diverse behaviors, we need to uncover the molecular mechanisms of within-cell-type functional heterogeneity. While single-cell RNA sequencing (scRNAseq) has advanced our understanding of cell heterogeneity, linking individual cell phenotypes to transcriptomic data remains challenging. Here, we used a phenotypic cell sorting technique to ask whether phenotypically supervised scRNAseq analysis (pheno-scRNAseq) can provide more insight into heterogeneous cell behaviors than unsupervised scRNAseq. Using a simple 3D in vitro breast cancer (BRCA) model, we conducted pheno-scRNAseq on invasive and non-invasive cells and compared the results to phenotype-agnostic scRNAseq analysis. Pheno-scRNAseq identified unique and more selective differentially expressed genes than unsupervised scRNAseq analysis. Functional studies validated the utility of pheno-scRNAseq in understanding within-cell-type functional heterogeneity and revealed that migration phenotypes were coordinated with specific metabolic, proliferation, stress, and immune phenotypes. This approach lends new insight into the molecular systems underlying BRCA cell phenotypic heterogeneity.
Keywords: Cell Biology; Complex System Biology; Transcriptomics.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chappell L., Russell A.J.C., Voet T. Single-cell (Multi)omics technologies. Annu. Rev. Genomics Hum. Genet. 2018;19:15–41. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

