Interferon Regulatory Factor-5 in Resident Macrophage Promotes Polycystic Kidney Disease
- PMID: 33490963
- PMCID: PMC7822573
- DOI: 10.34067/KID.0001052019
Interferon Regulatory Factor-5 in Resident Macrophage Promotes Polycystic Kidney Disease
Abstract
Background: Autosomal dominant polycystic kidney disease is caused by genetic mutations in PKD1 or PKD2. Macrophages and their associated inflammatory cytokines promote cyst progression; however, transcription factors within macrophages that control cytokine production and cystic disease are unknown.
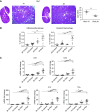
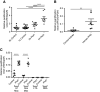
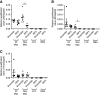
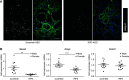
Methods: In these studies, we used conditional Pkd1 mice to test the hypothesis that macrophage-localized interferon regulatory factor-5 (IRF5), a transcription factor associated with production of cyst-promoting cytokines (TNFα, IL-6), is required for accelerated cyst progression in a unilateral nephrectomy (1K) model. Analyses of quantitative real-time PCR (qRT-PCR) and flow-cytometry data 3 weeks post nephrectomy, a time point before the onset of severe cystogenesis, indicate an accumulation of inflammatory infiltrating and resident macrophages in 1K Pkd1 mice compared with controls. qRT-PCR data from FACS cells at this time demonstrate that macrophages from 1K Pkd1 mice have increased expression of Irf5 compared with controls. To determine the importance of macrophage-localized Irf5 in cyst progression, we injected scrambled or IRF5 antisense oligonucleotide (ASO) in 1K Pkd1 mice and analyzed the effect on macrophage numbers, cytokine production, and renal cystogenesis 6 weeks post nephrectomy.
Results: Analyses of qRT-PCR and IRF5 ASO treatment significantly reduced macrophage numbers, Irf5 expression in resident-but not infiltrating-macrophages, and the severity of cystic disease. In addition, IRF5 ASO treatment in 1K Pkd1 mice reduced Il6 expression in resident macrophages, which was correlated with reduced STAT3 phosphorylation and downstream p-STAT3 target gene expression.
Conclusions: These data suggest that Irf5 promotes inflammatory cytokine production in resident macrophages resulting in accelerated cystogenesis.
Conflict of interest statement
M. Mrug has received research/clinical trial support from and has consulted for Otsuka and Sanofi-Genzyme. The remaining authors have nothing to disclose.
Figures


References
-
- Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van’t Hoff WG, Niaudet P, Torres VE, Harris PC, Harris PC: Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease [published correction appears in Kidney Int 75: 1359, 2009]. Kidney Int 75: 848–855, 2009 - PMC - PubMed
-
- Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ: Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- P30 DK090868/DK/NIDDK NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- R03 DK119717/DK/NIDDK NIH HHS/United States
- I01 BX002298/BX/BLRD VA/United States
- I01 BX004232/BX/BLRD VA/United States
- K08 DK106465/DK/NIDDK NIH HHS/United States
- U01 DA045300/DA/NIDA NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- U54 DK126114/DK/NIDDK NIH HHS/United States
- R01 DK097423/DK/NIDDK NIH HHS/United States
- R01 DK115752/DK/NIDDK NIH HHS/United States
- R01 DK065655/DK/NIDDK NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

