Impact of donor and recipient Epstein-Barr Virus serostatus on outcomes of allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis
- PMID: 33491135
- PMCID: PMC7914248
- DOI: 10.1007/s00277-021-04428-9
Impact of donor and recipient Epstein-Barr Virus serostatus on outcomes of allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis
Abstract
Allogeneic hematopoietic cell transplant (allo-HCT) is a potentially curative therapeutic strategy that showed encouraging long-term outcomes in hematological diseases. A number of factors can influence post-transplant clinical outcomes. While Epstein-Barr virus (EBV) constitutes a trigger for development of various adverse conditions, no clinical study yet has been powered to assess the effect of EBV serostatus on the clinical outcomes in allo-HCT population. To systematically summarize and analyze the impact of donor and recipient EBV serostatus on transplant outcomes in allo-HCT recipients, meta-analyses were conducted. Selected endpoints were overall survival (OS), relapse-free survival (RFS), relapse incidence (RI), non-relapse mortality (NRM), acute graft-versus-host disease (aGVHD), chronic graft-versus-host disease (cGVHD), and de novo cGVHD. Three studies with 26,650 patients, transplanted for acute leukemias, lymphomas, chronic hematological malignancies, or non-malignant hematological diseases were included in the meta-analysis. In the whole population, with a total of 53,300 donors and recipients, the rate of EBV seropositivity was 85.1%, including 86.6% and 83.6% among transplant recipients and healthy donors, respectively. Donor EBV seropositivity increased the risk of cGVHD by 17%, de novo cGVHD by 14%, and aGHVD by 5%. Recipient EBV seropositivity increased the risk of cGVHD by 12%, de novo cGVHD by 17%; increased NRM by 11%, increased RI by 11%, decreased OS by 14%, and decreased RFS by 11%. In performed meta-analyses, donor and recipient EBV seropositivity was found to have a significant impact on transplant outcomes in patients after allo-HCT.
Keywords: EBV; Epstein-Barr virus; GVHD; Graft-versus-host disease; HCT; Hematopoietic cell transplantation; Non-relapse mortality; Overall survival; Relapse incidence; Relapse-free survival.
Conflict of interest statement
The authors declare that they have no conflict of interest.
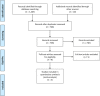
Figures







Similar articles
-
Prognostic impact of EBV serostatus in patients with lymphomas or chronic malignancies undergoing allogeneic HCT.Bone Marrow Transplant. 2019 Dec;54(12):2060-2071. doi: 10.1038/s41409-019-0627-9. Epub 2019 Jul 30. Bone Marrow Transplant. 2019. PMID: 31363166
-
Donor-Recipient Matching for KIR Genotypes Reduces Chronic GVHD and Missing Inhibitory KIR Ligands Protect against Relapse after Myeloablative, HLA Matched Hematopoietic Cell Transplantation.PLoS One. 2016 Jun 24;11(6):e0158242. doi: 10.1371/journal.pone.0158242. eCollection 2016. PLoS One. 2016. PMID: 27341514 Free PMC article.
-
Pilot Study of Donor-Engrafted Clonal Hematopoiesis Evolution and Clinical Outcomes in Allogeneic Hematopoietic Cell Transplantation Recipients Using a National Registry.Transplant Cell Ther. 2023 Oct;29(10):640.e1-640.e8. doi: 10.1016/j.jtct.2023.07.021. Epub 2023 Jul 28. Transplant Cell Ther. 2023. PMID: 37517612 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Apr 20;12(1):246. doi: 10.1186/s13287-021-02304-x. Stem Cell Res Ther. 2021. PMID: 33879242 Free PMC article.
Cited by
-
How I prevent viral reactivation in high-risk patients.Blood. 2023 Apr 27;141(17):2062-2074. doi: 10.1182/blood.2021014676. Blood. 2023. PMID: 36493341 Free PMC article. Review.
-
Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation.Microorganisms. 2022 Oct 27;10(11):2128. doi: 10.3390/microorganisms10112128. Microorganisms. 2022. PMID: 36363720 Free PMC article.
-
Clinical value of plasma and peripheral blood mononuclear cells Epstein-Barr Virus DNA dynamics on prognosis of allogeneic stem cell transplantation.Front Cell Infect Microbiol. 2022 Sep 16;12:980113. doi: 10.3389/fcimb.2022.980113. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189344 Free PMC article.
-
Exploring the Human Virome: Composition, Dynamics, and Implications for Health and Disease.Curr Microbiol. 2023 Nov 25;81(1):16. doi: 10.1007/s00284-023-03537-0. Curr Microbiol. 2023. PMID: 38006423 Review.
-
Exploring the role of Epstein-Barr virus infection on the clinical features and survival in locally advanced cervical cancer: a retrospective cohort study.Front Oncol. 2024 Dec 24;14:1522244. doi: 10.3389/fonc.2024.1522244. eCollection 2024. Front Oncol. 2024. PMID: 39777354 Free PMC article.
References
-
- Styczynski J. Managing post-transplant lymphoproliferative disorder. Exp Opin Orphan Drugs. 2017;5(1):19–35. doi: 10.1080/21678707.2017.1262256. - DOI
-
- Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, Ljungman P. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101(7):803–811. doi: 10.3324/haematol.2016.144428. - DOI - PMC - PubMed
-
- Sundin M, Le Blanc K, Ringden O, Barkholt L, Omazic B, Lergin C, Levitsky V, Remberger M. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91(8):1059–1067. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

