Synthesis of [18F]PS13 and Evaluation as a PET Radioligand for Cyclooxygenase-1 in Monkey
- PMID: 33491441
- PMCID: PMC9021884
- DOI: 10.1021/acschemneuro.0c00737
Synthesis of [18F]PS13 and Evaluation as a PET Radioligand for Cyclooxygenase-1 in Monkey
Abstract
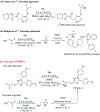
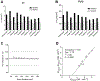
Cyclooxygenase-1 (COX-1) and its isozyme COX-2 are key enzymes in the syntheses of prostanoids. Imaging of COX-1 and COX-2 selective radioligands with positron emission tomography (PET) may clarify how these enzymes are involved in inflammatory conditions and assist in the discovery of improved anti-inflammatory drugs. We have previously labeled the selective high-affinity COX-1 ligand, 1,5-bis(4-methoxyphenyl)-3-(2,2,2-trifluoroethoxy)-1H-1,2,4-triazole (PS13), with carbon-11 (t1/2 = 20.4 min). This radioligand ([11C]PS13) has been successful for PET imaging of COX-1 in monkey and human brain and in periphery. [11C]PS13 is being used in clinical investigations. Alternative labeling of PS13 with fluorine-18 (t1/2 = 109.8 min) is desirable to provide a longer-lived radioligand in high activity that might be readily distributed among imaging centers. However, labeling of PS13 in its 1,1,1-trifluoroethoxy group is a radiochemical challenge. Here we assess two labeling approaches based on nucleophilic addition of cyclotron-produced [18F]fluoride ion to gem-difluorovinyl precursors, either to label PS13 in one step or to produce [18F]2,2,2-trifluoroethyl p-toluenesulfonate for labeling a hydroxyl precursor. From the latter two-step approach, we obtained [18F]PS13 ready for intravenous injection in a decay-corrected radiochemical yield of 7.9% and with a molar activity of up to 7.9 GBq/μmol. PET imaging of monkey brain with [18F]PS13 shows that this radioligand can specifically image and quantify COX-1 without radiodefluorination but with some radioactivity uptake in skull, ascribed to red bone marrow. The development of a new procedure for labeling PS13 with fluorine-18 at a higher molar activity is, however, desirable to suppress occupancy of COX-1 by carrier at baseline.
Keywords: COX-1; Fluorine-18; PET; PS13; brain imaging; nucleophilic addition; radioligand.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


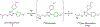

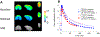

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

