Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer
- PMID: 33491448
- PMCID: PMC7931446
- DOI: 10.1021/acs.accounts.0c00591
Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer
Abstract
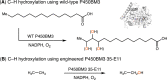
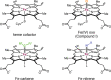
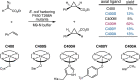
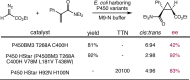
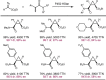
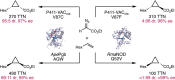
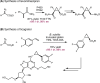
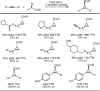
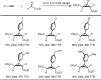
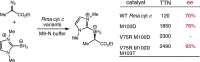
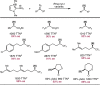
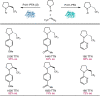
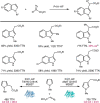
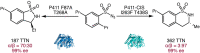
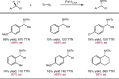
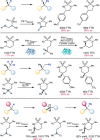
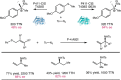
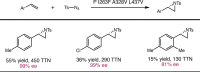
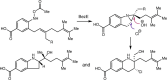
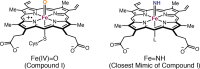
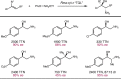
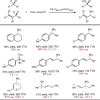
Despite the astonishing diversity of naturally occurring biocatalytic processes, enzymes do not catalyze many of the transformations favored by synthetic chemists. Either nature does not care about the specific products, or if she does, she has adopted a different synthetic strategy. In many cases, the appropriate reagents used by synthetic chemists are not readily accessible to biological systems. Here, we discuss our efforts to expand the catalytic repertoire of enzymes to encompass powerful reactions previously known only in small-molecule catalysis: formation and transfer of reactive carbene and nitrene intermediates leading to a broad range of products, including products with bonds not known in biology. In light of the structural similarity of iron carbene (Fe═C(R1)(R2)) and iron nitrene (Fe═NR) to the iron oxo (Fe═O) intermediate involved in cytochrome P450-catalyzed oxidation, we have used synthetic carbene and nitrene precursors that biological systems have not encountered and repurposed P450s to catalyze reactions that are not known in the natural world. The resulting protein catalysts are fully genetically encoded and function in intact microbial cells or cell-free lysates, where their performance can be improved and optimized by directed evolution. By leveraging the catalytic promiscuity of P450 enzymes, we evolved a range of carbene and nitrene transferases exhibiting excellent activity toward these new-to-nature reactions. Since our initial report in 2012, a number of other heme proteins including myoglobins, protoglobins, and cytochromes c have also been found and engineered to promote unnatural carbene and nitrene transfer. Due to the altered active-site environments, these heme proteins often displayed complementary activities and selectivities to P450s.Using wild-type and engineered heme proteins, we and others have described a range of selective carbene transfer reactions, including cyclopropanation, cyclopropenation, Si-H insertion, B-H insertion, and C-H insertion. Similarly, a variety of asymmetric nitrene transfer processes including aziridination, sulfide imidation, C-H amidation, and, most recently, C-H amination have been demonstrated. The scopes of these biocatalytic carbene and nitrene transfer reactions are often complementary to the state-of-the-art processes based on small-molecule transition-metal catalysts, making engineered biocatalysts a valuable addition to the synthetic chemist's toolbox. Moreover, enabled by the exquisite regio- and stereocontrol imposed by the enzyme catalyst, this biocatalytic platform provides an exciting opportunity to address challenging problems in modern synthetic chemistry and selective catalysis, including ones that have eluded synthetic chemists for decades.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
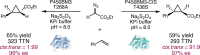


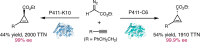






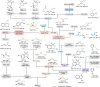
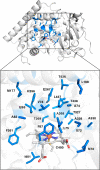
References
-
- Chen K.; Arnold F. H. Engineering New Catalytic Activities in Enzymes. Nat. Catal. 2020, 3, 203–213. 10.1038/s41929-019-0385-5. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

