WNK4 kinase: from structure to physiology
- PMID: 33491560
- PMCID: PMC7988811
- DOI: 10.1152/ajprenal.00634.2020
WNK4 kinase: from structure to physiology
Abstract
With no lysine kinase-4 (WNK4) belongs to a serine-threonine kinase family characterized by the atypical positioning of its catalytic lysine. Despite the fact that WNK4 has been found in many tissues, the majority of its study has revolved around its function in the kidney, specifically as a positive regulator of the thiazide-sensitive NaCl cotransporter (NCC) in the distal convoluted tubule of the nephron. This is explained by the description of gain-of-function mutations in the gene encoding WNK4 that causes familial hyperkalemic hypertension. This disease is mainly driven by increased downstream activation of the Ste20/SPS1-related proline-alanine-rich kinase/oxidative stress responsive kinase-1-NCC pathway, which increases salt reabsorption in the distal convoluted tubule and indirectly impairs renal K+ secretion. Here, we review the large volume of information that has accumulated about different aspects of WNK4 function. We first review the knowledge on WNK4 structure and enumerate the functional domains and motifs that have been characterized. Then, we discuss WNK4 physiological functions based on the information obtained from in vitro studies and from a diverse set of genetically modified mouse models with altered WNK4 function. We then review in vitro and in vivo evidence on the different levels of regulation of WNK4. Finally, we go through the evidence that has suggested how different physiological conditions act through WNK4 to modulate NCC activity.
Keywords: blood pressure; distal convoluted tubule; distal nephron; epithelial transport; familial hyperkalemic hypertension; potassium.
Figures




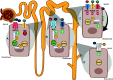



References
-
- Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi:10.1126/science.1062844. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

