Nrf2 mediates hypoxia-inducible HIF1α activation in kidney tubular epithelial cells
- PMID: 33491566
- PMCID: PMC7988808
- DOI: 10.1152/ajprenal.00501.2020
Nrf2 mediates hypoxia-inducible HIF1α activation in kidney tubular epithelial cells
Abstract
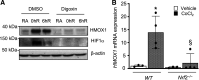
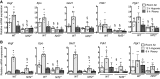
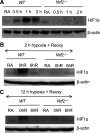
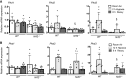
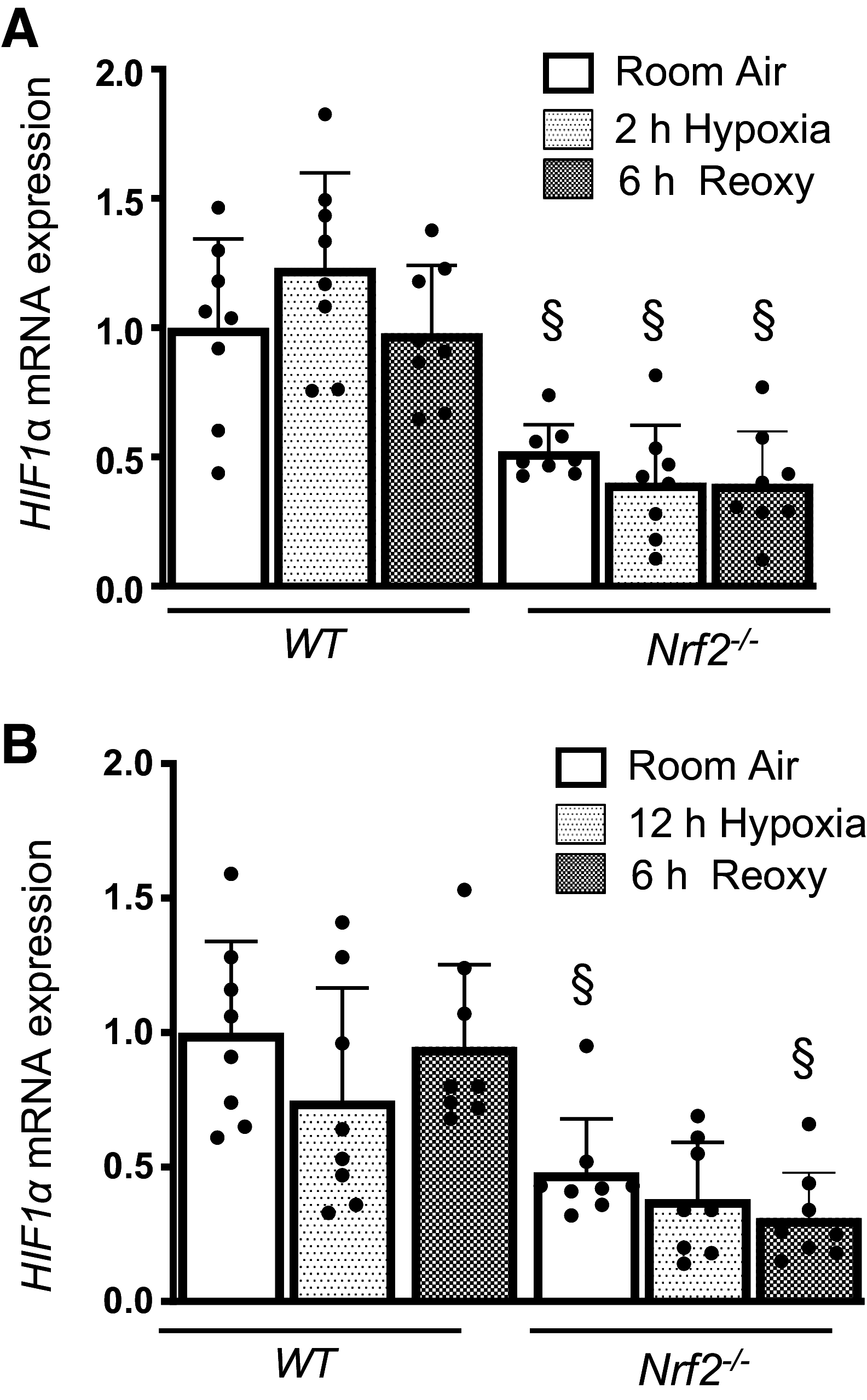
Nuclear factor erythroid 2-related factor 2 (Nrf2) and hypoxia-inducible factor-1α (HIF1α) transcription factors protect against ischemic acute kidney injury (AKI) by upregulating metabolic and cytoprotective gene expression. In this study, we tested the hypothesis that Nrf2 is required for HIF1α-mediated hypoxic responses using Nrf2-sufficient (wild-type) and Nrf2-deficient (Nrf2-/-) primary murine renal/kidney tubular epithelial cells (RTECs) and human immortalized tubular epithelial cells (HK2 cells) with HIF1 inhibition and activation. The HIF1 pathway inhibitor digoxin blocked hypoxia-stimulated HIF1α activation and heme oxygenase (HMOX1) expression in HK2 cells. Hypoxia-mimicking cobalt (II) chloride-stimulated HMOX1 expression was significantly lower in Nrf2-/- RTECs than in wild-type counterparts. Similarly, hypoxia-stimulated HIF1α-dependent metabolic gene expression was markedly impaired in Nrf2-/- RTECs. Nrf2 deficiency impaired hypoxia-induced HIF1α stabilization independent of increased prolyl 4-hydroxylase gene expression. We found decreased HIF1α mRNA levels in Nrf2-/- RTECs under both normoxia and hypoxia-reoxygenation conditions. In silico analysis and chromatin immunoprecipitation assays demonstrated Nrf2 binding to the HIF1α promoter in normoxia, but its binding decreased in hypoxia-exposed HK2 cells. However, Nrf2 binding at the HIF1α promoter was enriched following reoxygenation, demonstrating that Nrf2 maintains constitutive HIF1α expression. Consistent with this result, we found decreased levels of Nrf2 in hypoxia and that were restored following reoxygenation. Inhibition of mitochondrial complex I prevented hypoxia-induced Nrf2 downregulation and also increased basal Nrf2 levels. These results demonstrate a crucial role for Nrf2 in optimal HIF1α activation in hypoxia and that mitochondrial signaling downregulates Nrf2 levels in hypoxia, whereas reoxygenation restores it. Nrf2 and HIF1α interact to provide optimal metabolic and cytoprotective responses in ischemic AKI.
Keywords: acute kidney injury; hypoxia-inducible factor-1α; ischemia-reperfusion; mitochondria; nuclear factor erythroid 2-related factor 2.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Nrf2-AKT interactions regulate heme oxygenase 1 expression in kidney epithelia during hypoxia and hypoxia-reoxygenation.Am J Physiol Renal Physiol. 2016 Nov 1;311(5):F1025-F1034. doi: 10.1152/ajprenal.00362.2016. Epub 2016 Aug 31. Am J Physiol Renal Physiol. 2016. PMID: 27582105 Free PMC article.
-
Reoxygenation induces reactive oxygen species production and ferroptosis in renal tubular epithelial cells by activating aryl hydrocarbon receptor.Mol Med Rep. 2021 Jan;23(1):41. doi: 10.3892/mmr.2020.11679. Epub 2020 Nov 12. Mol Med Rep. 2021. PMID: 33179104 Free PMC article.
-
Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):3657-3663. doi: 10.1080/21691401.2019.1657879. Artif Cells Nanomed Biotechnol. 2019. Update in: Artif Cells Nanomed Biotechnol. 2021 Dec;49(1):676. doi: 10.1080/21691401.2021.2003046. PMID: 31478766 Updated.
-
In vivo survival strategies for cellular adaptation to hypoxia: HIF1α-dependent suppression of mitochondrial oxygen consumption and decrease of intracellular hypoxia are critical for survival of hypoxic chondrocytes.Bone. 2020 Nov;140:115572. doi: 10.1016/j.bone.2020.115572. Epub 2020 Aug 5. Bone. 2020. PMID: 32768687 Free PMC article. Review.
-
HIF1α and metabolic reprogramming in inflammation.J Clin Invest. 2016 Oct 3;126(10):3699-3707. doi: 10.1172/JCI84431. Epub 2016 Aug 29. J Clin Invest. 2016. PMID: 27571407 Free PMC article. Review.
Cited by
-
Involvement of Ferroptosis in Diabetes-Induced Liver Pathology.Int J Mol Sci. 2022 Aug 18;23(16):9309. doi: 10.3390/ijms23169309. Int J Mol Sci. 2022. PMID: 36012572 Free PMC article.
-
Insights into the Molecular Mechanisms of NRF2 in Kidney Injury and Diseases.Int J Mol Sci. 2023 Mar 23;24(7):6053. doi: 10.3390/ijms24076053. Int J Mol Sci. 2023. PMID: 37047024 Free PMC article. Review.
-
Sanguinarine chloride induces ferroptosis by regulating ROS/BACH1/HMOX1 signaling pathway in prostate cancer.Chin Med. 2024 Jan 9;19(1):7. doi: 10.1186/s13020-024-00881-6. Chin Med. 2024. PMID: 38195593 Free PMC article.
-
Hypoxia Pathways in Parkinson's Disease: From Pathogenesis to Therapeutic Targets.Int J Mol Sci. 2024 Sep 29;25(19):10484. doi: 10.3390/ijms251910484. Int J Mol Sci. 2024. PMID: 39408813 Free PMC article. Review.
-
The effect of HIF on metabolism and immunity.Nat Rev Nephrol. 2022 Sep;18(9):573-587. doi: 10.1038/s41581-022-00587-8. Epub 2022 Jun 20. Nat Rev Nephrol. 2022. PMID: 35726016 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

