TRIM37 prevents formation of centriolar protein assemblies by regulating Centrobin
- PMID: 33491649
- PMCID: PMC7870141
- DOI: 10.7554/eLife.62640
TRIM37 prevents formation of centriolar protein assemblies by regulating Centrobin
Abstract
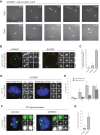
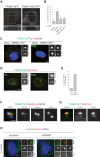
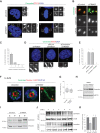
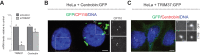
TRIM37 is an E3 ubiquitin ligase mutated in Mulibrey nanism, a disease with impaired organ growth and increased tumor formation. TRIM37 depletion from tissue culture cells results in supernumerary foci bearing the centriolar protein Centrin. Here, we characterize these centriolar protein assemblies (Cenpas) to uncover the mechanism of action of TRIM37. We find that an atypical de novo assembly pathway can generate Cenpas that act as microtubule-organizing centers (MTOCs), including in Mulibrey patient cells. Correlative light electron microscopy reveals that Cenpas are centriole-related or electron-dense structures with stripes. TRIM37 regulates the stability and solubility of Centrobin, which accumulates in elongated entities resembling the striped electron dense structures upon TRIM37 depletion. Furthermore, Cenpas formation upon TRIM37 depletion requires PLK4, as well as two parallel pathways relying respectively on Centrobin and PLK1. Overall, our work uncovers how TRIM37 prevents Cenpas formation, which would otherwise threaten genome integrity.
Keywords: CLEM; Centriole; Mulibrey nanism; TRIM37 E3 ligase; cell biology; centrobin; human; microtubule organizing center.
© 2021, Balestra et al.
Conflict of interest statement
FB, AD, BW, CB, AB, TA, ML, PH, RR, PG No competing interests declared
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

