The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease
- PMID: 33491853
- PMCID: PMC8359457
- DOI: 10.1002/alz.12283
The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease
Abstract
Introduction: This study investigated the diagnostic and disease-monitoring potential of plasma biomarkers in mild cognitive impairment (MCI) and Alzheimer's disease (AD) dementia and cognitively unimpaired (CU) individuals.
Methods: Plasma was analyzed using Simoa assays from 99 CU, 107 MCI, and 103 AD dementia participants.
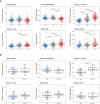
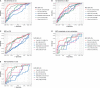
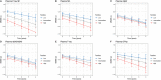
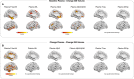
Results: Phosphorylated-tau181 (P-tau181), neurofilament light, amyloid-β (Aβ42/40), Total-tau and Glial fibrillary acidic protein were altered in AD dementia but P-tau181 significantly outperformed all biomarkers in differentiating AD dementia from CU (area under the curve [AUC] = 0.91). P-tau181 was increased in MCI converters compared to non-converters. Higher P-tau181 was associated with steeper cognitive decline and gray matter loss in temporal regions. Longitudinal change of P-tau181 was strongly associated with gray matter loss in the full sample and with Aβ measures in CU individuals.
Discussion: P-tau181 detected AD at MCI and dementia stages and was strongly associated with cognitive decline and gray matter loss. These findings highlight the potential value of plasma P-tau181 as a non-invasive and cost-effective diagnostic and prognostic biomarker in AD.
Keywords: Alzheimer's disease; magnetic resonance imaging; phosphorylated tau; plasma biomarkers.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Figures
References
-
- Ashton NJ, Scholl M, Heurling K, et al. Update on biomarkers for amyloid pathology in Alzheimer's disease. Biomark Med. 2018;12(7):799‐812. - PubMed
-
- Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet Neurol. 2014;13(6):614‐629. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

