Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics
- PMID: 33492118
- PMCID: PMC7838004
- DOI: 10.14309/ctg.0000000000000308
Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics
Abstract
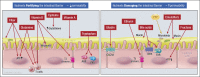
The objectives of this article are to understand the effects of stressors (nonsteroidal antiinflammatory drug, exercise, and pregnancy) and components in the diet, specifically prebiotics and probiotics, on intestinal barrier function. Stressors generally reduce barrier function, and these effects can be reversed by supplements such as zinc or glutamine that are among the substances that enhance the barrier. Other dietary factors in the diet that improve the barrier are vitamins A and D, tryptophan, cysteine, and fiber; by contrast, ethanol, fructose, and dietary emulsifiers increase permeability. Effects of prebiotics on barrier function are modest; on the other hand, probiotics exert direct and indirect antagonism of pathogens, and there are documented effects of diverse probiotic species, especially combination agents, on barrier function in vitro, in vivo in animal studies, and in human randomized controlled trials conducted in response to stress or disease. Clinical observations of benefits with combination probiotics in inflammatory diseases have simultaneously not appraised effects on intestinal permeability. In summary, probiotics and synbiotics enhance intestinal barrier function in response to stressor or disease states. Future studies should address the changes in barrier function and microbiota concomitant with assessment of clinical outcomes.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 2010;22:718–33. - PubMed
-
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. - PubMed
-
- Strocchi A, Levitt MD. A reappraisal of the magnitude and implications of the intestinal unstirred layer. Gastroenterology 1991;101:843–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

