PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures
- PMID: 33492447
- PMCID: PMC10992887
- DOI: 10.1007/s00262-021-02849-z
PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures
Abstract
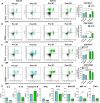
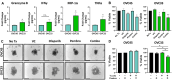
Immune checkpoint inhibitors (ICIs) that target programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) have shown modest activity as monotherapies for the treatment of ovarian cancer (OC). The rationale for using these therapies in combination with poly (ADP-ribose) polymerase inhibitors (PARP-Is) has been described, and their in vivo application will benefit from ex vivo platforms that aid in the prediction of patient response or resistance to therapy. This study examined the effectiveness of detecting patient-specific immune-related activity in OC using three-dimensional (3D) spheroids. Immune-related cell composition and PD-1/PD-L1 expression status were evaluated using cells dissociated from fresh OC tissue from two patients prior to and following 3D culture. The patient sample with the greatest increase in the proportion of PD-L1 + cells also possessed more activated cytotoxic T cells and mature DCs compared to the other patient sample. Upon cytokine stimulation, patient samples demonstrated increases in cytotoxic T cell activation and DC major histocompatibility complex (MHC) class-II expression. Pembrolizumab increased cytokine secretion, enhanced olaparib cytotoxicity, and reduced spheroid viability in a T cell-dependent manner. Furthermore, durvalumab and olaparib combination treatment increased cell death in a synergistic manner. This work demonstrates that immune cell activity and functional modulation can be accurately detected using our ex vivo 3D spheroid platform, and it presents evidence for their utility to demonstrate sensitivity to ICIs alone or in combination with PARP-Is in a preclinical setting.
Keywords: 3D cultures; Immune checkpoint inhibitors; Ovarian cancer; PARP inhibitors; Spheroid.
Conflict of interest statement
Dr. Appleton, Ms. Elrod, Ms. Lassahn, Mr. Shuford, Ms. Holmes, and Dr. DesRochers are current employees of KIYATEC, Inc.
Figures





References
-
- Pantelidou C, Sonzogni O, De Oliveria TM, Mehta AK, Kothari A, Wang D, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9(6):722–737. doi: 10.1158/2159-8290.CD-18-1218. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

