A New Era in Pharmacovigilance: Toward Real-World Data and Digital Monitoring
- PMID: 33492663
- PMCID: PMC8058244
- DOI: 10.1002/cpt.2172
A New Era in Pharmacovigilance: Toward Real-World Data and Digital Monitoring
Abstract
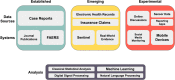
Adverse drug reactions (ADRs) are a major concern for patients, clinicians, and regulatory agencies. The discovery of serious ADRs leading to substantial morbidity and mortality has resulted in mandatory phase IV clinical trials, black box warnings, and withdrawal of drugs from the market. Real-world data, data collected during routine clinical care, is being adopted by innovators, regulators, payors, and providers to inform decision making throughout the product life cycle. We outline several different approaches to modern pharmacovigilance, including spontaneous reporting databases, electronic health record monitoring and research frameworks, social media surveillance, and the use of digital devices. Some of these platforms are well-established while others are still emerging or experimental. We highlight both the potential opportunity, as well as the existing challenges within these pharmacovigilance systems that have already begun to impact the drug development process, as well as the landscape of postmarket drug safety monitoring. Further research and investment into different and complementary pharmacovigilance systems is needed to ensure the continued safety of pharmacotherapy.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Giacomini, K.M. et al. When good drugs go bad. Nature 446, 975–977 (2007). - PubMed
-
- Pinnow, E. et al. Postmarket safety outcomes for new molecular entity (NME) drugs approved by the Food and Drug Administration between 2002 and 2014. Clin. Pharmacol. Ther. 104, 390–400 (2018). - PubMed
-
- Lester, J. et al. Evaluation of FDA safety‐related drug label changes in 2010. Pharmacoepidemiol. Drug Saf. 22, 302–305 (2013). - PubMed
-
- Platt, R. et al. The FDA Sentinel Initiative—an evolving national resource. N. Engl. J. Med. 379, 2091–2093 (2018). - PubMed
-
- Center for Drug Evaluation Research FDA D.I.S.C.O. Avelumab in Merkel Cell Carcinoma Transcript <https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐disco...> (2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

