Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis
- PMID: 33492719
- PMCID: PMC8048632
- DOI: 10.1111/jth.15246
Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis
Abstract
Background: A prolonged prothrombin time (PT) is a common feature in sepsis indicating consumptive coagulopathy.
Objectives: To determine the association between a prolonged PT and aberrations in other host response mechanisms in sepsis.
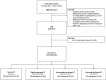
Methods: Patients admitted to the intensive care unit with sepsis were divided in quartiles according to the highest PT value measured within 24 h after admission. The host response was evaluated by measuring 19 plasma biomarkers reflecting pathways implicated in sepsis pathogenesis and by blood leukocyte gene expression profiling.
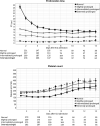
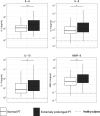
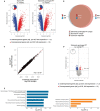
Measurements and main results: Of 1524 admissions for sepsis, 386 (25.3%) involved patients with a normal PT (≤12.7 s); the remaining quartiles entailed 379 (24.9%) patients with a slightly prolonged PT (12.8 ≤ PT ≤ 15.0 s), 383 (25.1%) with an intermediately prolonged PT (15.1 ≤ PT ≤ 17.2 s), and 376 (24.7%) with an extremely prolonged PT (≥17.3 s). While patients with an extremely prolonged PT showed an increased crude mortality up to 1 year after admission, none of the prolonged PT groups was independently associated with 30-day adjusted mortality. Comparison of the host response between patients with a normal PT or an extremely prolonged PT matched for baseline characteristics including severity of disease showed that an extremely prolonged PT was associated with impaired anticoagulant mechanisms, a more disturbed endothelial barrier integrity and increased systemic inflammation, and blood leukocyte transcriptomes indicating more prominent metabolic reprogramming and protein catabolism.
Conclusion: A prolonged PT is associated with stronger anomalies in pathways implicated in the pathogenesis of sepsis, suggesting that activation of coagulation impacts other host response mechanisms.
Keywords: endothelium inflammation; host response; intensive care unit; prothrombin time; sepsis.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
All authors have read and approved the manuscript. None of the authors declare any conflicts of interest.
Figures


References
-
- Chakraverty R, Davidson S, Peggs K, Stross P, Garrard C, Littlewood TJ The incidence and cause of coagulopathies in an intensive care population. Br J Haematol. 1996;93:460‐463. - PubMed
-
- Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33:341‐348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

