Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders
- PMID: 33492728
- PMCID: PMC8719798
- DOI: 10.1111/1751-7915.13750
Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders
Abstract
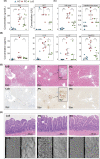
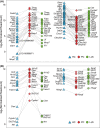
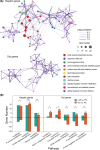
Millions of people die from liver diseases annually, and liver failure is one of the three major outcomes of liver disease. The gut microbiota plays a crucial role in liver diseases. This study aimed to explore the effects of Lactobacillus casei strain Shirota (LcS), a probiotics used widely around the world, on acute liver injury (ALI), as well as the underlying mechanism. Sprague Dawley rats were intragastrically administered LcS suspensions or placebo once daily for 7 days before induction of ALI by intraperitoneal injection of D-galactosamine (D-GalN). Histopathological examination and assessments of liver biochemical markers, inflammatory cytokines, and the gut microbiota, metabolome and transcriptome were conducted. Our results showed that pretreatment with LcS reduced hepatic and intestinal damage and reduced the elevation of serum gamma-glutamyltranspeptidase (GGT), total bile acids, IL-5, IL-10, G-CSF and RANTES. The analysis of the gut microbiota, metabolome and transcriptome showed that LcS lowered the ratio of Firmicutes to Bacteroidetes; reduced the enrichment of metabolites such as chenodeoxycholic acid, deoxycholic acid, lithocholic acid, d-talose and N-acetyl-glucosamine, reduce the depletion of d-glucose and l-methionine; and alleviated the downregulation of retinol metabolism and PPAR signalling and the upregulation of the pyruvate metabolism pathway in the liver. These results indicate the promising prospect of using LcS for the treatment of liver diseases, particularly ALI.
© 2021 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Lactobacillus reuteri DSM 17938 alleviates d-galactosamine-induced liver failure in rats.Biomed Pharmacother. 2021 Jan;133:111000. doi: 10.1016/j.biopha.2020.111000. Epub 2020 Nov 14. Biomed Pharmacother. 2021. PMID: 33202285
-
The oral administration of Lacticaseibacillus casei Shirota alleviates acetaminophen-induced liver injury through accelerated acetaminophen metabolism via the liver-gut axis in mice.mSphere. 2024 Jan 30;9(1):e0067223. doi: 10.1128/msphere.00672-23. Epub 2024 Jan 9. mSphere. 2024. PMID: 38193757 Free PMC article.
-
Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure.Eur J Nutr. 2016 Mar;55(2):821-831. doi: 10.1007/s00394-015-0904-3. Epub 2015 Apr 18. Eur J Nutr. 2016. PMID: 25893720
-
Probiotics as efficient immunopotentiators: translational role in cancer prevention.Indian J Med Res. 2013 Nov;138(5):808-14. Indian J Med Res. 2013. PMID: 24434333 Free PMC article. Review.
-
Biological effects of probiotics: what impact does Lactobacillus casei shirota have on us?Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1 Suppl):45S-50S. Int J Immunopathol Pharmacol. 2011. PMID: 21329565 Review.
Cited by
-
Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods.Front Microbiol. 2023 May 5;14:1170725. doi: 10.3389/fmicb.2023.1170725. eCollection 2023. Front Microbiol. 2023. PMID: 37213505 Free PMC article.
-
Apple Polyphenol Extract Suppresses Clostridioides difficile Infection in a Mouse Model.Metabolites. 2022 Oct 30;12(11):1042. doi: 10.3390/metabo12111042. Metabolites. 2022. PMID: 36355125 Free PMC article.
-
Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs.Int J Mol Sci. 2023 May 29;24(11):9444. doi: 10.3390/ijms24119444. Int J Mol Sci. 2023. PMID: 37298396 Free PMC article.
-
Effect of Galactooligosaccharide on PPARs/PI3K/Akt Pathway and Gut Microbiota in High-Fat and High-Sugar Diet Combined with STZ-Induced GDM Rat Model.Probiotics Antimicrob Proteins. 2025 Apr;17(2):888-902. doi: 10.1007/s12602-023-10186-z. Epub 2023 Nov 13. Probiotics Antimicrob Proteins. 2025. PMID: 37953344
-
From-Toilet-to-Freezer: A Review on Requirements for an Automatic Protocol to Collect and Store Human Fecal Samples for Research Purposes.Biomedicines. 2023 Sep 28;11(10):2658. doi: 10.3390/biomedicines11102658. Biomedicines. 2023. PMID: 37893032 Free PMC article. Review.
References
-
- Aoki, T. , Asahara, T. , Matsumoto, K. , Takada, T. , Chonan, O. , Nakamori, K. , et al. (2014) Effects of the continuous intake of a milk drink containing Lactobacillus casei strain Shirota on abdominal symptoms, fecal microbiota, and metabolites in gastrectomized subjects. Scand J Gastroenterol 49: 552–563. - PubMed
-
- Aoyagi, Y. , Amamoto, R. , Park, S. , Honda, Y. , Shimamoto, K. , Kushiro, A. , et al. (2019) independent and interactive effects of habitually ingesting fermented milk products containing lactobacillus Casei strain Shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front Microbiol 10: 1477. - PMC - PubMed
-
- Aoyagi, Y. , Park, S. , Matsubara, S. , Honda, Y. , Amamoto, R. , Kushiro, A. , et al. (2017) Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef Microbes 8: 23–29. - PubMed
-
- Cauli, O. , Lopez‐Larrubia, P. , Rodrigo, R. , Agusti, A. , Boix, J. , Nieto‐Charques, L. , et al. (2011) Brain region‐selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology 140: 638–645. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

