Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B-cell lymphoma
- PMID: 33492746
- PMCID: PMC7935786
- DOI: 10.1111/cas.14822
Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B-cell lymphoma
Abstract
Background: Tazemetostat is a selective and orally available inhibitor of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase and epigenetic regulator of cellular differentiation programs. We carried out a phase I study of tazemetostat in Japanese patients with relapsed or refractory B-cell non-Hodgkin-type lymphoma (B-NHL) to evaluate its tolerability, safety, pharmacokinetics, and preliminary antitumor activity.
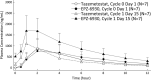
Methods: Tazemetostat was given orally at a single dose of 800 mg on the first day and 800 mg twice daily (BID: total 1600 mg/d) on following days in a 28-day/cycle manner. Tazemetostat dose-limiting toxicity (DLT) was evaluated up to the end of the first treatment cycle. Archival tumor tissues were analyzed for hotspot EZH2 mutations.
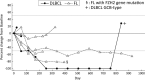
Results: As of 15 January 2018, seven patients (four follicular lymphoma [FL] and three diffuse large B-cell lymphoma [DLBCL]) were enrolled. The median age was 73 (range, 59-85) years, and the median number of prior chemotherapy regimens was three (range, one to five). No DLT was observed (one patient was not evaluable due to early disease progression). The common treatment-related adverse events (AEs) were thrombocytopenia and dysgeusia (three patients each; 42.9%). No treatment-related serious AEs were observed. The objective response rate was 57% (4/7 patients), including responses in three of four patients with FL and one of three patients with DLBCL. An EZH2 mutation was detected in one patient with FL responding to treatment.
Conclusions: Tazemetostat at 800 mg BID showed an acceptable safety profile and promising antitumor activity in Japanese patients with relapsed or refractory B-NHL.
Keywords: diffuse large B-cell lymphoma; enhancer of zeste homolog 2; follicular lymphoma; phase I study; tazemetostat.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare the following potential conflicts. KT: HUYA Bioscience, consultancy, honoraria; Bristol‐Myers Squibb, honoraria; Verastem, honoraria; Takeda Pharmaceutical, consultancy, honoraria, research funding; Eisai, honoraria, research funding; Kyowa Kirin, honoraria, research funding; Celgene, consultancy, honoraria, research funding; Zenyaku Kogyo, consultancy, honoraria; AbbVie, research funding; Yakult, honoraria; Janssen Pharmaceutical, honoraria, research funding; Mundi Pharma, consultancy, honoraria, research funding; Solasia, honoraria; Meiji Seika, honoraria; Daiichi Sankyo, consultancy, honoraria; Ono Pharmaceutical, consultancy, honoraria, research funding; Chugai Pharmaceutical, honoraria, research funding. SM: personal fees (BMS/Celgene, Chugai, Daiichi‐Sankyo, Eisai, Novartis, Symbio, Takeda). DM: personal fees and grant (Ono Pharmaceuticals, Celgene, Takeda Pharmaceutical, Janssen Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb), personal fees (Eisai, Kyowa Kirin, Zenyaku Kogyo Company, Synmosa Biopharma, Nippon Sinyaku), grant (Merck, Amgen Astellas BioPharma, Astellas Pharma, Sanofi, Novartis Pharma, Otsuka Pharmaceutical). KI: four honoraria and research funding (Eisai). TN, SS, SH: employees of Eisai Co., Ltd. KA: research funding (Eisai). The other authors have no conflict of interest.
Figures
Similar articles
-
Phase II study of tazemetostat for relapsed or refractory B-cell non-Hodgkin lymphoma with EZH2 mutation in Japan.Cancer Sci. 2021 Sep;112(9):3627-3635. doi: 10.1111/cas.15040. Epub 2021 Jul 14. Cancer Sci. 2021. PMID: 34159682 Free PMC article. Clinical Trial.
-
Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study.Lancet Oncol. 2018 May;19(5):649-659. doi: 10.1016/S1470-2045(18)30145-1. Epub 2018 Apr 9. Lancet Oncol. 2018. PMID: 29650362 Clinical Trial.
-
Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial.Lancet Oncol. 2020 Nov;21(11):1433-1442. doi: 10.1016/S1470-2045(20)30441-1. Epub 2020 Oct 6. Lancet Oncol. 2020. PMID: 33035457 Free PMC article. Clinical Trial.
-
A Matching-Adjusted Indirect Comparison of Single-Arm Trials in Patients with Relapsed or Refractory Follicular Lymphoma Who Received at Least Two Prior Systemic Treatments: Tazemetostat was Associated with a Lower Risk for Safety Outcomes Versus the PI3-Kinase Inhibitors Idelalisib, Duvelisib, Copanlisib, and Umbralisib.Adv Ther. 2022 Apr;39(4):1678-1696. doi: 10.1007/s12325-022-02054-z. Epub 2022 Feb 14. Adv Ther. 2022. PMID: 35157216 Free PMC article.
-
Pharmacology and pharmacokinetics of tazemetostat.Cancer Chemother Pharmacol. 2024 May;93(5):509-517. doi: 10.1007/s00280-024-04658-4. Epub 2024 Mar 23. Cancer Chemother Pharmacol. 2024. PMID: 38520556 Free PMC article. Review.
Cited by
-
Unraveling the Immune Microenvironment in Diffuse Large B-Cell Lymphoma: Prognostic and Potential Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 5;46(7):7048-7064. doi: 10.3390/cimb46070420. Curr Issues Mol Biol. 2024. PMID: 39057061 Free PMC article. Review.
-
Pharmacokinetics of Herb-Drug Interactions of Plumbagin and Tazemetostat in Rats by UPLC-MS/MS.Drug Des Devel Ther. 2022 Sep 29;16:3385-3394. doi: 10.2147/DDDT.S384156. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36199632 Free PMC article.
-
Calcium signalling pathways in prostate cancer initiation and progression.Nat Rev Urol. 2023 Sep;20(9):524-543. doi: 10.1038/s41585-023-00738-x. Epub 2023 Mar 24. Nat Rev Urol. 2023. PMID: 36964408 Review.
-
Quantitation of tazemetostat in human plasma using liquid chromatography-tandem mass spectrometry.Biomed Chromatogr. 2024 Jul;38(7):e5903. doi: 10.1002/bmc.5903. Epub 2024 May 23. Biomed Chromatogr. 2024. PMID: 38783541 Free PMC article.
-
Epigenetic alterations and advancement of lymphoma treatment.Ann Hematol. 2024 May;103(5):1435-1454. doi: 10.1007/s00277-023-05395-z. Epub 2023 Aug 15. Ann Hematol. 2024. PMID: 37581713 Review.
References
-
- Comet I, Riising EM, Leblanc B, et al. Maintaining cell identity: PRC2‐mediated regulation of transcription and cancer. Nat Rev Cancer. 2016;16:803‐810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

