Magnesium Oxide-Catalyzed Conversion of Chitin to Lactic Acid
- PMID: 33492785
- PMCID: PMC7953471
- DOI: 10.1002/open.202000303
Magnesium Oxide-Catalyzed Conversion of Chitin to Lactic Acid
Abstract
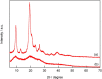
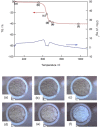
Although chitin, an N-acetyl-D-glucosamine polysaccharide, can be converted to valuable products by means of homogeneous catalysis, most of the chitin generated by food processing is treated as industrial waste. Thus, a method for converting this abundant source of biomass to useful chemicals, such as lactic acid, would be beneficial. In this study, we determined the catalytic activities of various metal oxides for chitin conversion at 533 K and found that MgO showed the highest activity for lactic acid production. X-ray diffraction analysis and thermogravimetry-differential thermal analysis showed that the MgO was transformed to Mg(OH)2 during chitin conversion. The highest yield of lactic acid (10.8 %) was obtained when the reaction was carried out for 6 h with 0.5 g of the MgO catalyst. The catalyst could be recovered as a solid residue after the reaction and reused twice with no decrease in the lactic acid yield.
Keywords: biomass; chitin conversion; heterogeneous catalysis; lactic acid; magnesium oxide.
© 2021 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hamed I., Özogul F., Regenstein J. M. Trends Food Sci. Technol. 2016, 48, 40–50.
-
- Knidri H. E., Belaabed R., Addaou A., Laajeb A., Lahsini A. Int. J. Biol. Macromol. 2018, 120, 1181–1189. - PubMed
-
- Kobayashi H., Ohta H., Fukuoka A. Catal. Sci. Technol. 2012, 2, 869–883.
-
- Agirrezabal-Telleria I., Gandarias I., Arias P. L. Catal. Today. 2014, 234, 42–58.
-
- Yamaguchi A., Sato O., Mimura N., Hirosaki Y., Kobayashi H., Fukuoka A., Shirai M. Catal. Commun. 2014, 54, 22–26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

