Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02)
- PMID: 33492986
- PMCID: PMC8078488
- DOI: 10.1200/JCO.20.02712
Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02)
Abstract
Purpose: As yet, no checkpoint inhibitor has been approved to treat nasopharyngeal carcinoma (NPC). This study was aimed to evaluate the antitumor activity, safety, and biomarkers of toripalimab, a new programmed death-1 (PD-1) inhibitor for recurrent or metastatic NPC (RM-NPC) refractory to standard chemotherapy.
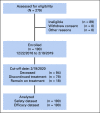
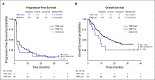
Patients and methods: In this single-arm, multicenter phase II study, patients with RM-NPC received 3 mg/kg toripalimab once every 2 weeks via intravenous infusion until confirmed disease progression or unacceptable toxicity. The primary end point was objective response rate (ORR). The secondary end points included safety, duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
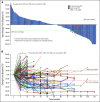
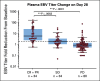
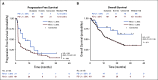
Results: Among all 190 patients, the ORR was 20.5% with median DOR 12.8 months, median PFS 1.9 months, and median OS 17.4 months. Among 92 patients who failed at least two lines of systemic chemotherapy, the ORR was 23.9%. The ORRs were 27.1% and 19.4% in PD-L1+ and PD-L1- patients, respectively (P = .31). Patients with ≥ 50% decrease of plasma Epstein-Barr virus (EBV) DNA copy number on day 28 had significantly better ORR than those with < 50% decrease, 48.3% versus 5.7% (P = .0001). Tumor mutational burden had a median value of 0.95 muts/mega-base in the cohort and had no predictive value for response. Whole-exome sequencing results from 174 patients revealed that the patients with genomic amplification in 11q13 region or ETV6 genomic alterations had poor responses to toripalimab.
Conclusion: The POLARIS-02 study demonstrated a manageable safety profile and durable clinical response of toripalimab in patients with chemorefractory metastatic NPC. An early decrease in plasma EBV DNA copy number correlated with favorable response.
Trial registration: ClinicalTrials.gov NCT02915432.
Figures

References
-
- Chen YP Chan ATC Le QT, et al. : Nasopharyngeal carcinoma. Lancet 394:64-80, 2019 - PubMed
-
- Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15:1765-17677, 2006 - PubMed
-
- Ou SI Zell JA Ziogas A, et al. : Epidemiology of nasopharyngeal carcinoma in the United States: Improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol 18:29-35, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

