Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19
- PMID: 33493012
- PMCID: PMC7863679
- DOI: 10.7326/M20-6739
Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19
Erratum in
-
Correction: Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19.Ann Intern Med. 2021 Jun;174(6):888. doi: 10.7326/L21-0148. Ann Intern Med. 2021. PMID: 34126034 No abstract available.
Abstract
Background: Hypercoagulability may be a key mechanism of death in patients with coronavirus disease 2019 (COVID-19).
Objective: To evaluate the incidence of venous thromboembolism (VTE) and major bleeding in critically ill patients with COVID-19 and examine the observational effect of early therapeutic anticoagulation on survival.
Design: In a multicenter cohort study of 3239 critically ill adults with COVID-19, the incidence of VTE and major bleeding within 14 days after intensive care unit (ICU) admission was evaluated. A target trial emulation in which patients were categorized according to receipt or no receipt of therapeutic anticoagulation in the first 2 days of ICU admission was done to examine the observational effect of early therapeutic anticoagulation on survival. A Cox model with inverse probability weighting to adjust for confounding was used.
Setting: 67 hospitals in the United States.
Participants: Adults with COVID-19 admitted to a participating ICU.
Measurements: Time to death, censored at hospital discharge, or date of last follow-up.
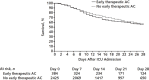
Results: Among the 3239 patients included, the median age was 61 years (interquartile range, 53 to 71 years), and 2088 (64.5%) were men. A total of 204 patients (6.3%) developed VTE, and 90 patients (2.8%) developed a major bleeding event. Independent predictors of VTE were male sex and higher D-dimer level on ICU admission. Among the 2809 patients included in the target trial emulation, 384 (11.9%) received early therapeutic anticoagulation. In the primary analysis, during a median follow-up of 27 days, patients who received early therapeutic anticoagulation had a similar risk for death as those who did not (hazard ratio, 1.12 [95% CI, 0.92 to 1.35]).
Limitation: Observational design.
Conclusion: Among critically ill adults with COVID-19, early therapeutic anticoagulation did not affect survival in the target trial emulation.
Primary funding source: None.
Conflict of interest statement
Figures


Comment in
-
[Anticoagulation in SARS-CoV-2 infection: state of the art and management in patients with home treatment].Rev Chilena Infectol. 2021 Jun;38(3):461-462. doi: 10.4067/S0716-10182021000300461. Rev Chilena Infectol. 2021. PMID: 34479307 Spanish. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
