Pupillary reflex and behavioral masking responses to light as functional measures of retinal degeneration in mice
- PMID: 33493166
- PMCID: PMC7833141
- DOI: 10.1371/journal.pone.0244702
Pupillary reflex and behavioral masking responses to light as functional measures of retinal degeneration in mice
Abstract
Background: Pre-clinical testing of retinal pathology and treatment efficacy depends on reliable and valid measures of retinal function. The electroretinogram (ERG) and tests of visual acuity are the ideal standard, but can be unmeasurable while useful vision remains. Non-image-forming responses to light such as the pupillary light reflex (PLR) are attractive surrogates. However, it is not clear how accurately such responses reflect changes in visual capability in specific disease models. The purpose of this study was to test whether measures of non-visual responses to light correlate with previously determined visual function in two photoreceptor degenerations.
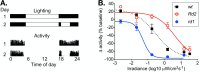
Methods: The sensitivity of masking behavior (light induced changes in running wheel activity) and the PLR were measured in 3-month-old wild-type mice (WT) with intact inner retinal circuitry, Pde6b-rd1/rd1 mice (rd1) with early and rapid loss of rods and cones, and Prph2-Rd2/Rd2 mice (Rd2) with a slower progressive loss of rods and cones.
Results: In rd1 mice, negative masking had increased sensitivity, positive masking was absent, and the sensitivity of the PLR was severely reduced. In Rd2 mice, positive masking identified useful vision at higher light levels, but there was a limited decrease in the irradiance sensitivity of negative masking and the PLR, and the amplitude of change for both underestimated the reduction in irradiance sensitivity of image-forming vision.
Conclusions: Together these data show that in a given disease, two responses to light can be affected in opposite ways, and that for a given response to light, the change in the response does not accurately represent the degree of pathology. However, the extent of the deficit in the PLR means that even a limited rescue of rod/cone function might be measured by increased PLR amplitude. In addition, positive masking has the potential to measure effective treatment in both models by restoring responses or shifting thresholds to lower irradiances.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Prusky GT, West PWR, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40: 2201–2209. Available: www.elsevier.com/locate/visres 10.1016/s0042-6989(00)00081-x - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

