Manganese exposure in juvenile C57BL/6 mice increases glial inflammatory responses in the substantia nigra following infection with H1N1 influenza virus
- PMID: 33493177
- PMCID: PMC7833173
- DOI: 10.1371/journal.pone.0245171
Manganese exposure in juvenile C57BL/6 mice increases glial inflammatory responses in the substantia nigra following infection with H1N1 influenza virus
Abstract
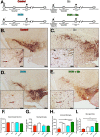
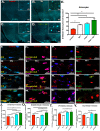
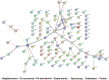
Infection with Influenza A virus can lead to the development of encephalitis and subsequent neurological deficits ranging from headaches to neurodegeneration. Post-encephalitic parkinsonism has been reported in surviving patients of H1N1 infections, but not all cases of encephalitic H1N1 infection present with these neurological symptoms, suggesting that interactions with an environmental neurotoxin could promote more severe neurological damage. The heavy metal, manganese (Mn), is a potential interacting factor with H1N1 because excessive exposure early in life can induce long-lasting effects on neurological function through inflammatory activation of glial cells. In the current study, we used a two-hit model of neurotoxin-pathogen exposure to examine whether exposure to Mn during juvenile development would induce a more severe neuropathological response following infection with H1N1 in adulthood. To test this hypothesis, C57BL/6 mice were exposed to MnCl2 in drinking water (50 mg/kg/day) for 30 days from days 21-51 postnatal, then infected intranasally with H1N1 three weeks later. Analyses of dopaminergic neurons, microglia and astrocytes in basal ganglia indicated that although there was no significant loss of dopaminergic neurons within the substantia nigra pars compacta, there was more pronounced activation of microglia and astrocytes in animals sequentially exposed to Mn and H1N1, as well as altered patterns of histone acetylation. Whole transcriptome Next Generation Sequencing (RNASeq) analysis was performed on the substantia nigra and revealed unique patterns of gene expression in the dual-exposed group, including genes involved in antioxidant activation, mitophagy and neurodegeneration. Taken together, these results suggest that exposure to elevated levels of Mn during juvenile development could sensitize glial cells to more severe neuro-immune responses to influenza infection later in life through persistent epigenetic changes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Beal MF. Parkinson’s disease: a model dilemma. Nature. 2010;466(7310). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

