The ARREST Pneumonia Clinical Trial. Rationale and Design
- PMID: 33493423
- PMCID: PMC8008996
- DOI: 10.1513/AnnalsATS.202009-1115SD
The ARREST Pneumonia Clinical Trial. Rationale and Design
Abstract
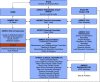
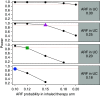
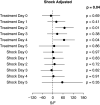
Patients hospitalized for pneumonia are at high risk for mortality. Effective therapies are therefore needed. Recent randomized clinical trials suggest that systemic steroids can reduce the length of hospital stays among patients hospitalized for pneumonia. Furthermore, preliminary findings from a feasibility study demonstrated that early treatment with a combination of an inhaled corticosteroid and a bronchodilator can improve oxygenation and reduce risk of respiratory failure in patients at risk of acute respiratory distress syndrome. Whether such a combination administered early is effective in reducing acute respiratory failure (ARF) among patients hospitalized with pneumonia is unknown. Here we describe the ARREST Pneumonia (Arrest Respiratory Failure due to Pneumonia) trial designed to address this question. ARREST Pneumonia is a two-arm, randomized, double-blinded, placebo-controlled trial designed to test the efficacy of a combination of an inhaled corticosteroid and a β-agonist compared with placebo for the prevention of ARF in hospitalized participants with severe pneumonia. The primary outcome is ARF within 7 days of randomization, defined as a composite endpoint of intubation and mechanical ventilation; need for high-flow nasal cannula oxygen therapy or noninvasive ventilation for >36 hours (each alone or combined); or death within 36 hours of being placed on respiratory support. The planned enrollment is 600 adult participants at 10 academic medical centers. In addition, we will measure selected plasma biomarkers to better understand mechanisms of action. The trial is funded by the U.S. National Heart Lung and Blood Institute.Clinical trial registered with www.clinicaltrials.gov (NCT04193878).
Keywords: aerosol drug therapy; pneumonia; prevention; respiratory failure.
Figures


References
-
- Pfuntner A, Wier LM, Steiner C. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2006. Costs for hospital stays in the United States, 2011: statistical brief #168. - PubMed
-
- Pfuntner A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. Most frequent conditions in US hospitals, 2011: statistical brief #162.
-
- Heron M. Deaths: leading causes for 2011. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2015;64:1–96. - PubMed
-
- Ewig S, Ruiz M, Mensa J, Marcos MA, Martinez JA, Arancibia F, et al. Severe community-acquired pneumonia: assessment of severity criteria. Am J Respir Crit Care Med. 1998;158:1102–1108. - PubMed
-
- Valencia M, Badia JR, Cavalcanti M, Ferrer M, Agustí C, Angrill J, et al. Pneumonia severity index class V patients with community-acquired pneumonia: characteristics, outcomes, and value of severity scores. Chest. 2007;132:515–522. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

