Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial
- PMID: 33493448
- PMCID: PMC7826120
- DOI: 10.1016/S2213-2600(20)30470-7
Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial
Abstract
Background: Mechanical ventilation in intensive care for 48 h or longer is associated with the acute respiratory distress syndrome (ARDS), which might be present at the time ventilatory support is instituted or develop afterwards, predominantly during the first 5 days. Survivors of prolonged mechanical ventilation and ARDS are at risk of considerably impaired physical function that can persist for years. An early pathogenic mechanism of lung injury in mechanically ventilated, critically ill patients is inflammation-induced pulmonary fibrin deposition, leading to thrombosis of the microvasculature and hyaline membrane formation in the air sacs. The main aim of this study was to determine if nebulised heparin, which targets fibrin deposition, would limit lung injury and thereby accelerate recovery of physical function in patients with or at risk of ARDS.
Methods: The Can Heparin Administration Reduce Lung Injury (CHARLI) study was an investigator-initiated, multicentre, double-blind, randomised phase 3 trial across nine hospitals in Australia. Adult intensive care patients on invasive ventilation, with impaired oxygenation defined by a PaO2/FiO2 ratio of less than 300, and with the expectation of invasive ventilation beyond the next calendar day were recruited. Key exclusion criteria were heparin allergy, pulmonary bleeding, and platelet count less than 50 X 109/L. Patients were randomly assigned 1:1, with stratification by site and using blocks of variable size and random seed, via a web-based system, to either unfractionated heparin sodium 25 000 IU in 5 mL or identical placebo (sodium chloride 0·9% 5 mL), administered using a vibrating mesh membrane nebuliser every 6 h to day 10 while invasively ventilated. Patients, clinicians, and investigators were masked to treatment allocation. The primary outcome was the Short Form 36 Health Survey Physical Function Score (out of 100) of survivors at day 60. Prespecified secondary outcomes, which are exploratory, included development of ARDS to day 5 among at-risk patients, deterioration of the Murray Lung Injury Score (MLIS) to day 5, mortality at day 60, residence of survivors at day 60, and serious adverse events. Analyses followed the intention-to-treat principle. There was no imputation of missing data. The trial is registered with the Australian and New Zealand Clinical Trials Register, number ACTRN12612000418875 .
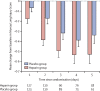
Findings: Between Sept 4, 2012, and Aug 23, 2018, 256 patients were randomised. Final follow-up was on Feb 25, 2019. We excluded three patients who revoked consent and one ineligible participant who received no intervention. Of 252 patients included in data analysis, the mean age was 58 years (SD 15), 157 (62%) were men, and 118 (47%) had ARDS. 128 (51%) patients were assigned to the heparin group and 124 (49%) to the placebo group, all of whom received their assigned intervention. Survivors in the heparin group (n=97) had similar SF-36 Physical Function Scores at day 60 compared to the placebo group (n=94; mean 53·6 [SD 31·6] vs 48·7 [35·7]; difference 4·9 [95% CI -4·8 to 14·5]; p=0·32). Compared with the placebo group, the heparin group had fewer cases of ARDS develop to day 5 among the at-risk patients (nine [15%] of 62 patients vs 21 [30%] of 71 patients; hazard ratio 0·46 [95% CI 0·22 to 0·98]; p=0·0431), less deterioration of the MLIS to day 5 (difference -0·14 [-0·26 to -0·02]; p=0·0215), similar day 60 mortality (23 [18%] of 127 patients vs 18 [15%] of 123 patients; odds ratio [OR] 1·29 [95% CI 0·66 to 2·53]; p=0·46), and more day 60 survivors at home (86 [87%] of 99 patients vs 73 [73%] of 100 patients; OR 2·45 [1·18 to 5·08]; p=0·0165). A similar number of serious adverse events occurred in each group (seven [5%] of 128 patients in the heparin group vs three [2%] of 124 patients in the placebo group; OR 2·33 [0·59 to 9·24]; p=0·23), which were a transient increase in airway pressure during nebulisation (n=3 in the heparin group), major non-pulmonary bleeding (n=2 in each group), haemoptysis (n=1 in the heparin group), tracheotomy site bleeding (n=1 in the heparin group), and hypoxaemia during nebulisation (n=1 in the placebo group).
Interpretation: In patients with or at risk of ARDS, nebulised heparin did not improve self-reported performance of daily physical activities, but was well tolerated and exploratory outcomes suggest less progression of lung injury and earlier return home. Further research is justified to establish if nebulised heparin accelerates recovery in those who have or are at risk of ARDS.
Funding: Rowe Family Foundation, TR and RB Ditchfield Medical Research Endowment Fund, Patricia Madigan Charitable Trust, and The J and R McGauran Trust Fund.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Nebulised heparin for patients on ventilation: implications for COVID-19 pneumonia.Lancet Respir Med. 2021 Apr;9(4):321-322. doi: 10.1016/S2213-2600(20)30513-0. Epub 2021 Jan 22. Lancet Respir Med. 2021. PMID: 33493452 Free PMC article. No abstract available.
References
-
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. - PubMed
-
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. - PubMed
-
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. - PubMed
-
- Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. - PubMed
-
- Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

