A new, unquenched intermediate of LHCII
- PMID: 33493515
- PMCID: PMC7949128
- DOI: 10.1016/j.jbc.2021.100322
A new, unquenched intermediate of LHCII
Abstract
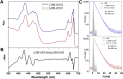
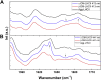
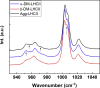
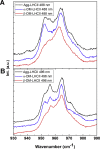
When plants are exposed to high-light conditions, the potentially harmful excess energy is dissipated as heat, a process called non-photochemical quenching. Efficient energy dissipation can also be induced in the major light-harvesting complex of photosystem II (LHCII) in vitro, by altering the structure and interactions of several bound cofactors. In both cases, the extent of quenching has been correlated with conformational changes (twisting) affecting two bound carotenoids, neoxanthin, and one of the two luteins (in site L1). This lutein is directly involved in the quenching process, whereas neoxanthin senses the overall change in state without playing a direct role in energy dissipation. Here we describe the isolation of an intermediate state of LHCII, using the detergent n-dodecyl-α-D-maltoside, which exhibits the twisting of neoxanthin (along with changes in chlorophyll-protein interactions), in the absence of the L1 change or corresponding quenching. We demonstrate that neoxanthin is actually a reporter of the LHCII environment-probably reflecting a large-scale conformational change in the protein-whereas the appearance of excitation energy quenching is concomitant with the configuration change of the L1 carotenoid only, reflecting changes on a smaller scale. This unquenched LHCII intermediate, described here for the first time, provides for a deeper understanding of the molecular mechanism of quenching.
Keywords: LHCII; NPQ; light-harvesting complex; photoprotection; resonance Raman.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Scholes G.D., Fleming G.R., Olaya-Castro A., van Grondelle R. Lessons from nature about solar light harvesting. Nat. Chem. 2011;3:763–774. - PubMed
-
- Croce R., van Amerongen H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science. 2020;369 - PubMed
-
- Niyogi K.K., Truong T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013;16:307–314. - PubMed
-
- Pinnola A., Bassi R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018;46:467–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

