CUEDC2 ablation enhances the efficacy of mesenchymal stem cells in ameliorating cerebral ischemia/reperfusion insult
- PMID: 33494071
- PMCID: PMC7906146
- DOI: 10.18632/aging.202394
CUEDC2 ablation enhances the efficacy of mesenchymal stem cells in ameliorating cerebral ischemia/reperfusion insult
Abstract
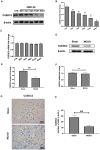
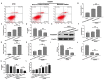
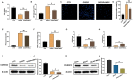
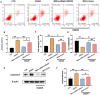
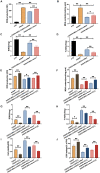
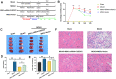
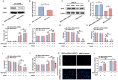
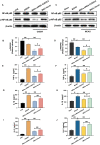
Mesenchymal stem cell (MSC) therapy has been reported to be a promising therapeutic option for cerebral ischemia/reperfusion (I/R) insult. However, the poor survival rate of engrafted MSCs under unfavorable cerebral I/R-induced microenvironment inhibits their efficiency during clinical application. CUE domain-containing 2(CUECD2) exhibits its protective role on cardiomyocytes by mediating the antioxidant capacity. Our study explored the functional role of CUEDC2 in cerebral I/R challenge and determined whether CUECD2-modified MSCs could improve the efficacy of treatment of the insulted neurons. We also evaluated the possible mechanisms involved in cerebral I/R condition. Cerebral I/R stimulation suppressed CUEDC2 levels in brain tissues and neurons. siRNA-CUEDC2 in neurons significantly inhibited cerebral I/R-induced apoptosis and oxidative stress levels invitro. Moreover, siRNA-CUEDC2 in the MSCs group remarkably enhanced the therapeutic efficacies in cerebral I/R-induced neuron injury and brain tissue impairment when compared to the non-genetic MSCs treatment group. At the molecular level, siRNA-CUEDC2 in MSCs markedly enhanced its antioxidant and anti-inflammatory effect in co-cultured neurons by upregulating glutathione peroxidase 1 (GPX1) expression levels while suppressing NF-kB activation. These findings provide a novel strategy for the utilization of MSCs to promote cerebral ischemic stroke outcomes.
Keywords: CUEDC2; MCAO; MSCs; OGD/R.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

