An Electrochemical Amperometric Ethylene Sensor with Solid Polymer Electrolyte Based on Ionic Liquid
- PMID: 33494275
- PMCID: PMC7864481
- DOI: 10.3390/s21030711
An Electrochemical Amperometric Ethylene Sensor with Solid Polymer Electrolyte Based on Ionic Liquid
Abstract
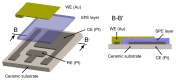
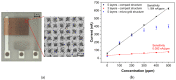
An electrochemical amperometric ethylene sensor with solid polymer electrolyte (SPE) and semi-planar three electrode topology involving a working, pseudoreference, and counter electrode is presented. The polymer electrolyte is based on the ionic liquid 1-butyl 3-methylimidazolium bis(trifluoromethylsulfonyl)imide [BMIM][NTf2] immobilized in a poly(vinylidene fluoride) matrix. An innovative aerosol-jet printing technique was used to deposit the gold working electrode (WE) on the solid polymer electrolyte layer to make a unique electrochemical active SPE/WE interface. The analyte, gaseous ethylene, was detected by oxidation at 800 mV vs. the platinum pseudoreference electrode. The sensor parameters such as sensitivity, response/recovery time, repeatability, hysteresis, and limits of detection and quantification were determined and their relation to the morphology and microstructure of the SPE/WE interface examined. The use of additive printing techniques for sensor preparation demonstrates the potential of polymer electrolytes with respect to the mass production of printed electrochemical gas sensors.
Keywords: ethylene; ionic liquid; printed electrochemical sensor; solid polymer electrolyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Solvent Evaporation Rate as a Tool for Tuning the Performance of a Solid Polymer Electrolyte Gas Sensor.Polymers (Basel). 2022 Nov 6;14(21):4758. doi: 10.3390/polym14214758. Polymers (Basel). 2022. PMID: 36365751 Free PMC article.
-
An Electrochemical NO₂ Sensor Based on Ionic Liquid: Influence of the Morphology of the Polymer Electrolyte on Sensor Sensitivity.Sensors (Basel). 2015 Nov 11;15(11):28421-34. doi: 10.3390/s151128421. Sensors (Basel). 2015. PMID: 26569248 Free PMC article.
-
A Printed and Flexible NO2 Sensor Based on a Solid Polymer Electrolyte.Front Chem. 2019 Apr 26;7:286. doi: 10.3389/fchem.2019.00286. eCollection 2019. Front Chem. 2019. PMID: 31080794 Free PMC article.
-
Continuous amperometric hydrogen gas sensing in ionic liquids.Analyst. 2018 Aug 20;143(17):4136-4146. doi: 10.1039/c8an00577j. Analyst. 2018. PMID: 30065973
-
Gel Electrolytes for Electrochemical Actuators and Sensors Applications.Chem Asian J. 2023 Feb 1;18(3):e202201160. doi: 10.1002/asia.202201160. Epub 2023 Jan 2. Chem Asian J. 2023. PMID: 36537994 Review.
Cited by
-
Printed Platinum Nanoparticle Thin-Film Structures for Use in Biology and Catalysis: Synthesis, Printing, and Application Demonstration.ACS Omega. 2023 Jan 4;8(2):1929-1936. doi: 10.1021/acsomega.2c04687. eCollection 2023 Jan 17. ACS Omega. 2023. PMID: 36687057 Free PMC article.
-
Fullerene-Doped Poly(ionic liquids) as Small Molecular Gas Sensors-Control of Intermolecular Interactions.ACS Omega. 2024 Dec 23;10(1):1364-1372. doi: 10.1021/acsomega.4c08941. eCollection 2025 Jan 14. ACS Omega. 2024. PMID: 39829479 Free PMC article.
-
Impact of Initial Cyclic Loading on Mechanical Properties and Performance of Nafion.Sensors (Basel). 2023 Jan 29;23(3):1488. doi: 10.3390/s23031488. Sensors (Basel). 2023. PMID: 36772526 Free PMC article.
-
Solvent Evaporation Rate as a Tool for Tuning the Performance of a Solid Polymer Electrolyte Gas Sensor.Polymers (Basel). 2022 Nov 6;14(21):4758. doi: 10.3390/polym14214758. Polymers (Basel). 2022. PMID: 36365751 Free PMC article.
-
Additive Manufacturing of Organic Electrochemical Transistors: Methods, Device Architectures, and Emerging Applications.Small. 2025 Mar;21(11):e2410499. doi: 10.1002/smll.202410499. Epub 2025 Feb 13. Small. 2025. PMID: 39945058 Free PMC article. Review.
References
-
- Saltveit M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999;15:279–292. doi: 10.1016/S0925-5214(98)00091-X. - DOI
-
- FAO . Global Food Losses and Food Waste—Extent, Causes and Prevention. FAO; Rome, Italy: 2011.
-
- FAO . The State of Food and Agriculture 2019-Moving Forward on Food Loss and Waste Reduce. FAO; Rome, Italy: 2019.
-
- Chauhan R., Moreno M., Banda D.M., Zamborini F.P., Grapperhaus C.A. Chemiresistive metal-stabilized thiyl radical films as highly selective ethylene sensors. RSC Adv. 2014;4:46787–46790. doi: 10.1039/C4RA07560A. - DOI
-
- Li B., Li M., Meng F., Liu J. Highly sensitive ethylene sensors using Pd nanoparticles and rGO modified flower-like hierarchical porous Α-Fe2O3. Sens. Actuators B Chem. 2019;290:396–405. doi: 10.1016/j.snb.2019.04.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

