Protein Loading into Spongelike PLGA Microspheres
- PMID: 33494293
- PMCID: PMC7909807
- DOI: 10.3390/pharmaceutics13020137
Protein Loading into Spongelike PLGA Microspheres
Abstract
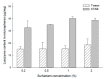
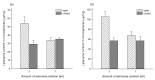
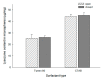
A self-healing microencapsulation process involves mixing preformed porous microspheres in an aqueous solution containing the desired protein and converting them into closed-pore microspheres. Spongelike poly-d,l-lactide-co-glycolide (PLGA) microspheres are expected to be advantageous to protein loading through self-healing. This study aimed to identify and assess relevant critical parameters, using lysozyme as a model protein. Several parameters governed lysozyme loading. The pore characteristics (open-pore, closed-pore, and porosity) of the preformed microspheres substantially affected lysozyme loading efficiency. The type of surfactant present in the aqueous medium also influenced lysozyme loading efficiency. For instance, cetyltrimethylammonium bromide showing a superior wetting functionality increased the extent of lysozyme loading more than twice as compared to Tween 80. Dried preformed microspheres were commonly used before, but our study found that wet microspheres obtained at the end of the microsphere manufacturing process displayed significant advantages in lysozyme loading. Not only could an incubation time for hydrating the microspheres be shortened dramatically, but also a much more considerable amount of lysozyme was encapsulated. Interestingly, the degree of microsphere hydration determined the microstructure and morphology of closed-pore microspheres after self-healing. Understanding these critical process parameters would help tailor protein loading into spongelike PLGA microspheres in a bespoke manner.
Keywords: closed-pore; microencapsulation; open-pore; poly-d,l-lactide-co-glycolide; porous microspheres; protein.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures










References
-
- Bao T.Q., Hiep N.T., Kim Y.H., Yang H.M., Lee B.T. Fabrication and characterization of porous poly(lactic-co-glycolic acid) (PLGA) microspheres for use as a drug delivery system. J. Mater. Sci. 2011;46:2510–2517. doi: 10.1007/s10853-010-5101-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

