Natural and Synthetic Lactones Possessing Antitumor Activities
- PMID: 33494352
- PMCID: PMC7865919
- DOI: 10.3390/ijms22031052
Natural and Synthetic Lactones Possessing Antitumor Activities
Abstract
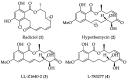
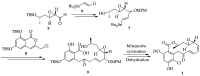
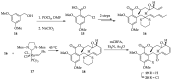
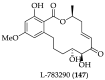
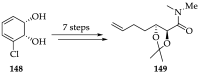
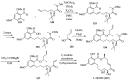
Cancer is one of the leading causes of death globally, accounting for an estimated 8 million deaths each year. As a result, there have been urgent unmet medical needs to discover novel oncology drugs. Natural and synthetic lactones have a broad spectrum of biological uses including anti-tumor, anti-helminthic, anti-microbial, and anti-inflammatory activities. Particularly, several natural and synthetic lactones have emerged as anti-cancer agents over the past decades. In this review, we address natural and synthetic lactones focusing on their anti-tumor activities and synthetic routes. Moreover, we aim to highlight our journey towards chemical modification and biological evaluation of a resorcylic acid lactone, L-783277 (4). We anticipate that utilization of the natural and synthetic lactones as novel scaffolds would benefit the process of oncology drug discovery campaigns based on natural products.
Keywords: anticancer activities; drug discovery; natural lactones; natural product synthesis; synthetic lactones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

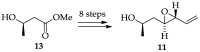

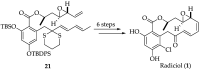

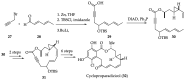
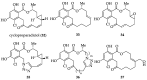

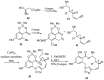
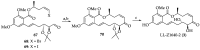
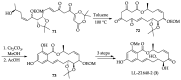
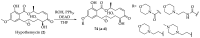
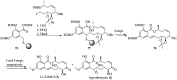
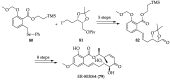
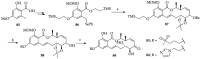
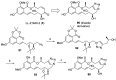
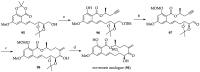
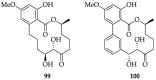
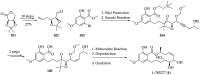
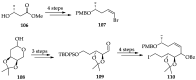
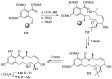

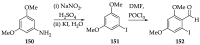







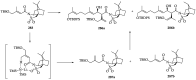
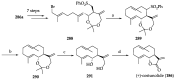
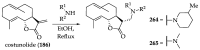
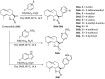
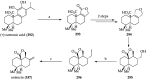
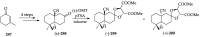
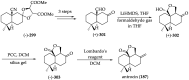
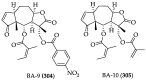


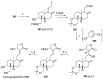
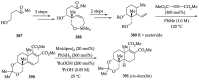
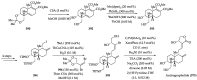
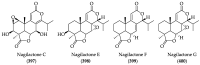

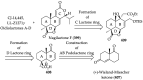
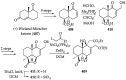
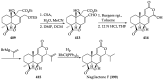
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

