Coal Fly Ash Derived Silica Nanomaterial for MMMs-Application in CO2/CH4 Separation
- PMID: 33494390
- PMCID: PMC7911253
- DOI: 10.3390/membranes11020078
Coal Fly Ash Derived Silica Nanomaterial for MMMs-Application in CO2/CH4 Separation
Abstract
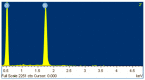
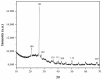
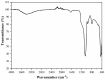
In order to obtained high selective membrane for industrial applications (such as natural gas purification), mixed matrix membranes (MMMs) were developed based on polysulfone as matrix and MCM-41-type silica material (obtained from coal fly ash) as filler. As a consequence, various quantities of filler were used to determine the membranes efficiency on CO2/CH4 separation. The coal fly ash derived silica nanomaterial and the membranes were characterized in terms of thermal stability, homogeneity, and pore size distribution. There were observed similar properties of the obtained nanomaterial with a typical MCM-41 (obtained from commercial silicates), such as high surface area and pore size distribution. The permeability tests highlighted that the synthesized membranes can be applicable for CO2 removal from CH4, due to unnoticeable differences between real and ideal selectivity. Additionally, the membranes showed high resistance to CO2 plasticization, due to permeability decrease even at high feed pressure, up to 16 bar.
Keywords: MMMs; coal fly ash; gas separation; mesoporous silica.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures













References
-
- Burmann P., Zornoza B., Tellez C., Coronas J. MMMs comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014;107:66–75. doi: 10.1016/j.ces.2013.12.001. - DOI
-
- Zhang Y., Sunarso J., Liu S., Wang R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas. Con. 2013;12:84–107. doi: 10.1016/j.ijggc.2012.10.009. - DOI
-
- Fernández-Barquín A., Casado-Costerillor C., Palomino M., Valencia S., Irabien A. Permselectivity improvement in membranes for CO2/N2 separation. Sep. Purif. Technol. 2016;157:102–111. doi: 10.1016/j.seppur.2015.11.032. - DOI
-
- Weng T.H., Tseng H.H., Wey M.Y. Fabrication and characterization of poly(phenylene oxide)/SBA-15/carbon molecule sieve multilayer mixed matrix membrane for gas separation. Int. J. Hydrogen Energy. 2010;35:6971–6983. doi: 10.1016/j.ijhydene.2010.04.024. - DOI
-
- Doong S.J. Membranes, adsorbent materials and solvent—Based materials for syngas and hydrogen separation. Funct. Mater. Sustain. Energy Appl. 2012;7:179–216. doi: 10.1533/9780857096371.2.179. - DOI
Grants and funding
- NUCLEU Program-Financing Contract no. 9N/2019, under Project PN 19 11 03/Romanian Ministry of Education and Research
- PN-III-P1-1.2-PCCDI-2017-0776/No. 36 PCCDI/15.03.2018/Romanian Ministry of Education and Research - UEFISCDI Authority
- Financial Agreement 51668/09.07.2019, SMIS code 124705/Ministry of European Funds - Operational Program Human Capital
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

