Decreased Levels of Thioredoxin o1 Influences Stomatal Development and Aperture but Not Photosynthesis under Non-Stress and Saline Conditions
- PMID: 33494429
- PMCID: PMC7865980
- DOI: 10.3390/ijms22031063
Decreased Levels of Thioredoxin o1 Influences Stomatal Development and Aperture but Not Photosynthesis under Non-Stress and Saline Conditions
Abstract
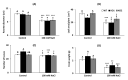
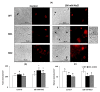
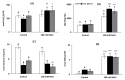
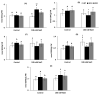
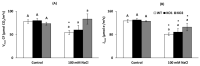
Salinity has a negative impact on plant growth, with photosynthesis being downregulated partially due to osmotic effect and enhanced cellular oxidation. Redox signaling contributes to the plant response playing thioredoxins (TRXs) a central role. In this work we explore the potential contribution of Arabidopsis TRXo1 to the photosynthetic response under salinity analyzing Arabidopsis wild-type (WT) and two Attrxo1 mutant lines in their growth under short photoperiod and higher light intensity than previous reported works. Stomatal development and apertures and the antioxidant, hormonal and metabolic acclimation are also analyzed. In control conditions mutant plants displayed less and larger developed stomata and higher pore size which could underlie their higher stomatal conductance, without being affected in other photosynthetic parameters. Under salinity, all genotypes displayed a general decrease in photosynthesis and the oxidative status in the Attrxo1 mutant lines was altered, with higher levels of H2O2 and NO but also higher ascorbate/glutathione (ASC/GSH) redox states than WT plants. Finally, sugar changes and increases in abscisic acid (ABA) and NO may be involved in the observed higher stomatal response of the TRXo1-altered plants. Therefore, the lack of AtTRXo1 affected stomata development and opening and the mutants modulate their antioxidant, metabolic and hormonal responses to optimize their adaptation to salinity.
Keywords: antioxidants; oxidative stress; photosynthesis; salinity; stomata; thioredoxin o1.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
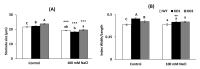




References
-
- Lázaro J.J., Jiménez A., Camejo D., Iglesias-Baena I., Martí M.C., Lázaro-Payo A., Barranco-Medina S., Sevilla F. Dissecting the integrative antioxidant and redox systems in plant mitochondria. Effect of stress and S-nitrosylation. Front. Plant Sci. 2013;4:1–20. doi: 10.3389/fpls.2013.00460. - DOI - PMC - PubMed
-
- Flexas J., Bota J., Galmés J., Medrano H., Ribas-Carbó M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006;127:343–352. doi: 10.1111/j.1399-3054.2006.00621.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

