DNA-Binding Anticancer Drugs: One Target, Two Actions
- PMID: 33494466
- PMCID: PMC7866126
- DOI: 10.3390/molecules26030552
DNA-Binding Anticancer Drugs: One Target, Two Actions
Abstract
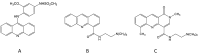
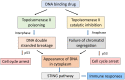
Amsacrine, an anticancer drug first synthesised in 1970 by Professor Cain and colleagues, showed excellent preclinical activity and underwent clinical trial in 1978 under the auspices of the US National Cancer Institute, showing activity against acute lymphoblastic leukaemia. In 1984, the enzyme DNA topoisomerase II was identified as a molecular target for amsacrine, acting to poison this enzyme and to induce DNA double-strand breaks. One of the main challenges in the 1980s was to determine whether amsacrine analogues could be developed with activity against solid tumours. A multidisciplinary team was assembled in Auckland, and Professor Denny played a leading role in this approach. Among a large number of drugs developed in the programme, N-[2-(dimethylamino)-ethyl]-acridine-4-carboxamide (DACA), first synthesised by Professor Denny, showed excellent activity against a mouse lung adenocarcinoma. It underwent clinical trial, but dose escalation was prevented by ion channel toxicity. Subsequent work led to the DACA derivative SN 28049, which had increased potency and reduced ion channel toxicity. Mode of action studies suggested that both amsacrine and DACA target the enzyme DNA topoisomerase II but with a different balance of cellular consequences. As primarily a topoisomerase II poison, amsacrine acts to turn the enzyme into a DNA-damaging agent. As primarily topoisomerase II catalytic inhibitors, DACA and SN 28049 act to inhibit the segregation of daughter chromatids during anaphase. The balance between these two actions, one cell cycle phase specific and the other nonspecific, together with pharmacokinetic, cytokinetic and immunogenic considerations, provides links between the actions of acridine derivatives and anthracyclines such as doxorubicin. They also provide insights into the action of cytotoxic DNA-binding drugs.
Keywords: DNA binding; antitumour; cell cycle; immunogenic cell death; pharmacokinetics; topoisomerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gatenby R.A., Brown J.S. Integrating evolutionary dynamics into cancer therapy. Nat. Rev. Clin. Oncol. 2020;17:675–686. - PubMed
-
- Zitvogel L., Apetoh L., Ghiringhelli F., Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008;8:59–73. - PubMed
-
- Watson J.D., Crick F.H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. - PubMed
-
- Cain B.F., Atwell G.J. The experimental antitumour properties of three congeners of the acridylmethanesulphonanilide (AMSA) series. Eur. J. Cancer Clin. Oncol. 1974;10:539–549. - PubMed
-
- Arlin Z.A. Current status of amsacrine (AMSA) combination chemotherapy programs in acute leukemia. Cancer Treat. Rep. 1983;67:967–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

