Therapeutic Sequencing in ALK+ NSCLC
- PMID: 33494549
- PMCID: PMC7912146
- DOI: 10.3390/ph14020080
Therapeutic Sequencing in ALK+ NSCLC
Abstract
Anaplastic lymphoma kinase-rearranged non-small-cell lung cancer (ALK+ NSCLC) is a model disease for the use of targeted pharmaceuticals in thoracic oncology. Due to higher systemic and intracranial efficacy, the second-generation ALK tyrosine kinase inhibitors (TKI) alectinib and brigatinib have irrevocably displaced crizotinib as standard first-line treatment, based on the results of the ALEX and ALTA-1L trials. Besides, lorlatinib and brigatinib are the preferred second-line therapies for progression under second-generation TKI and crizotinib, respectively, based on the results of several phase II studies. Tissue or liquid rebiopsies at the time of disease progression, even though not mandated by the approval status of any ALK inhibitor, are gaining importance for individualization and optimization of patient management. Of particular interest are cases with off-target resistance, for example MET, HER2 or KRAS alterations, which require special therapeutic maneuvers, e.g., inclusion in early clinical trials or off-label administration of respectively targeted drugs. On the other hand, up to approximately half of the patients failing TKI, develop anatomically restricted progression, which can be initially tackled with local ablative measures without switch of systemic therapy. Among the overall biologically favorable ALK+ tumors, with a mean tumor mutational burden uniquely below 3 mutations per Mb and the longest survival among NSCLC currently, presence of the EML4-ALK fusion variant 3 and/or TP53 mutations identify high-risk cases with earlier treatment failure and a need for more aggressive surveillance and treatment strategies. The potential clinical utility of longitudinal ctDNA assays for earlier detection of disease progression and improved guidance of therapy in these patients is a currently a matter of intense investigation. Major pharmaceutical challenges for the field are the development of more potent, fourth-generation TKI and effective immuno-oncological interventions, especially ALK-directed cell therapies, which will be essential for further improving survival and achieving cure of ALK+ tumors.
Keywords: ALK+ non-small-cell lung cancer; EML4-ALK fusion variant 3, chemotherapy; sequential therapies; tyrosine kinase inhibitors.
Conflict of interest statement
PC reports research funding from AstraZeneca, Novartis, Roche, Takeda, and advisory board/lecture/educational fees from AstraZeneca, Boehringer Ingelheim, Chugai, Kite, Novartis, Pfizer, Roche, Takeda. ME declares no conflict of interest. The funder had no role in the design of this work, in the collection and interpretation of the material presented, in the writing of the manuscript, or in the decision to publish it.
Figures
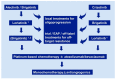
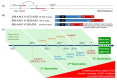
References
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., van Schil P.E., Hellmann M.D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. - DOI - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer, Version 1.2021. [(accessed on 14 December 2020)]; Available online: www.nccn.org.
-
- Selvaggi G., Wakelee H.A., Mok T., Wu Y.-L., Reck M., Chiappori A., Cicin I., Lee D.H., Breder V., Fan Y., et al. Abstract 2: Phase 3 Randomized Study of Ensartinib vs Crizotinib in Anaplastic Lymphoma Kinase (ALK)-Positive NSCLC Patients: eXalt3. In WCLC 2020 Virtual Presidential Symposium; Organized by the International Association for the Study of Lung Cancer (IASLC) [(accessed on 14 December 2020)]; Available online: https://wclc2020.iaslc.org/virtual-presidential-symposium.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

