Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI
- PMID: 33494815
- PMCID: PMC7831178
- DOI: 10.1186/s13054-020-03424-1
Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI
Abstract
Background: Sepsis is a life-threatening condition accompanied by organ dysfunction subsequent to a dysregulated host response to infection. Up to 60% of patients with sepsis develop acute kidney injury (AKI), which is associated with a poor clinical outcome. The pathophysiology of sepsis-associated AKI (sepsis-AKI) remains incompletely understood, but mitochondria have emerged as key players in the pathogenesis. Therefore, our aim was to identify mitochondrial damage in patients with sepsis-AKI.
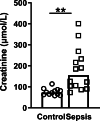
Methods: We conducted a clinical laboratory study using "warm" postmortem biopsies from sepsis-associated AKI patients from a university teaching hospital. Biopsies were taken from adult patients (n = 14) who died of sepsis with AKI at the intensive care unit (ICU) and control patients (n = 12) undergoing tumor nephrectomy. To define the mechanisms of the mitochondrial contribution to the pathogenesis of sepsis-AKI, we explored mRNA and DNA expression of mitochondrial quality mechanism pathways, DNA oxidation and mitochondrial DNA (mtDNA) integrity in renal biopsies from sepsis-AKI patients and control subjects. Next, we induced human umbilical vein endothelial cells (HUVECs) with lipopolysaccharide (LPS) for 48 h to mimic sepsis and validate our results in vitro.
Results: Compared to control subjects, sepsis-AKI patients had upregulated mRNA expression of oxidative damage markers, excess mitochondrial DNA damage and lower mitochondrial mass. Sepsis-AKI patients had lower mRNA expression of mitochondrial quality markers TFAM, PINK1 and PARKIN, but not of MFN2 and DRP1. Oxidative DNA damage was present in the cytosol of tubular epithelial cells in the kidney of sepsis-AKI patients, whereas it was almost absent in biopsies from control subjects. Oxidative DNA damage co-localized with both the nuclei and mitochondria. Accordingly, HUVECs induced with LPS for 48 h showed an increased mnSOD expression, a decreased TFAM expression and higher mtDNA damage levels.
Conclusion: Sepsis-AKI induces mitochondrial DNA damage in the human kidney, without upregulation of mitochondrial quality control mechanisms, which likely resulted in a reduction in mitochondrial mass.
Keywords: Acute kidney injury; Mitochondria; Reactive oxygen species; Sepsis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

