CAR T cells in solid tumors: challenges and opportunities
- PMID: 33494834
- PMCID: PMC7831265
- DOI: 10.1186/s13287-020-02128-1
CAR T cells in solid tumors: challenges and opportunities
Abstract
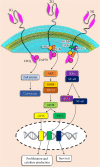
Background: CARs are simulated receptors containing an extracellular single-chain variable fragment (scFv), a transmembrane domain, as well as an intracellular region of immunoreceptor tyrosine-based activation motifs (ITAMs) in association with a co-stimulatory signal.
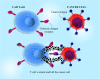
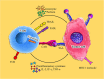
Main body: Chimeric antigen receptor (CAR) T cells are genetically engineered T cells to express a receptor for the recognition of the particular surface marker that has given rise to advances in the treatment of blood disorders. The CAR T cells obtain supra-physiological properties and conduct as "living drugs" presenting both immediate and steady effects after expression in T cells surface. But, their efficacy in solid tumor treatment has not yet been supported. The pivotal challenges in the field of solid tumor CAR T cell therapy can be summarized in three major parts: recognition, trafficking, and surviving in the tumor. On the other hand, the immunosuppressive tumor microenvironment (TME) interferes with T cell activity in terms of differentiation and exhaustion, and as a result of the combined use of CARs and checkpoint blockade, as well as the suppression of other inhibitor factors in the microenvironment, very promising results were obtained from the reduction of T cell exhaustion.
Conclusion: Nowadays, identifying and defeating the mechanisms associated with CAR T cell dysfunction is crucial to establish CAR T cells that can proliferate and lyse tumor cells severely. In this review, we discuss the CAR signaling and efficacy T in solid tumors and evaluate the most significant barriers in this process and describe the most novel therapeutic methods aiming to the acquirement of the promising therapeutic outcome in non-hematologic malignancies.
Keywords: CAR T cells; Cell therapy; Chimeric antigen receptor; Solid tumors.
Conflict of interest statement
There is no conflict of interests.
Figures


References
-
- Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

