Regional Brain Correlates of Beta Bursts in Health and Psychosis: A Concurrent Electroencephalography and Functional Magnetic Resonance Imaging Study
- PMID: 33495122
- PMCID: PMC8648891
- DOI: 10.1016/j.bpsc.2020.10.018
Regional Brain Correlates of Beta Bursts in Health and Psychosis: A Concurrent Electroencephalography and Functional Magnetic Resonance Imaging Study
Abstract
Background: There is emerging evidence for abnormal beta oscillations in psychosis. Beta oscillations are likely to play a key role in the coordination of sensorimotor information that is crucial to healthy mental function. Growing evidence suggests that beta oscillations typically manifest as transient beta bursts that increase in probability following a motor response, observable as post-movement beta rebound. Evidence indicates that post-movement beta rebound is attenuated in psychosis, with greater attenuation associated with greater symptom severity and impairment. Delineating the functional role of beta bursts therefore may be key to understanding the mechanisms underlying persistent psychotic illness.
Methods: We used concurrent electroencephalography and functional magnetic resonance imaging to identify blood oxygen level-dependent correlates of beta bursts during the n-back working memory task and intervening rest periods in healthy control participants (n = 30) and patients with psychosis (n = 48).
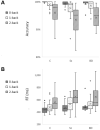
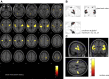
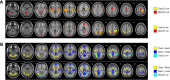
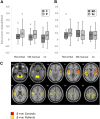
Results: During both task blocks and intervening rest periods, beta bursts phasically activated regions implicated in task-relevant content while suppressing currently tonically active regions. Patients showed attenuated post-movement beta rebound that was associated with persisting disorganization symptoms as well as impairments in cognition and role function. Patients also showed greater task-related reductions in overall beta burst rate and showed greater, more extensive, beta burst-related blood oxygen level-dependent activation.
Conclusions: Our evidence supports a model in which beta bursts reactivate latently maintained sensorimotor information and are dysregulated and inefficient in psychosis. We propose that abnormalities in the mechanisms by which beta bursts coordinate reactivation of contextually appropriate content can manifest as disorganization, working memory deficits, and inaccurate forward models and may underlie a core deficit associated with persisting symptoms and impairment.
Keywords: Concurrent EEG/fMRI; Persisting psychotic illness; Post-movement beta rebound; Psychosis; Transient beta oscillations; Working memory.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Beta Oscillations, Cognition, and Memory Function: Clues to the Nature of Psychosis?Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 Dec;6(12):1121. doi: 10.1016/j.bpsc.2021.08.004. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. PMID: 34887080 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

