Pretransplant Calculated Panel Reactive Antibody in the Absence of Donor-Specific Antibody and Kidney Allograft Survival
- PMID: 33495290
- PMCID: PMC7863647
- DOI: 10.2215/CJN.13640820
Pretransplant Calculated Panel Reactive Antibody in the Absence of Donor-Specific Antibody and Kidney Allograft Survival
Abstract
Background and objectives: Panel reactive antibody informs the likelihood of finding an HLA-compatible donor for transplant candidates, but has historically been associated with acute rejection and allograft survival because testing methods could not exclude the presence of concomitant donor-specific antibodies. Despite new methods to exclude donor-specific antibodies, panel reactive antibody continues to be used to determine the choice of induction and maintenance immunosuppression. The study objective was to determine the clinical relevance of panel reactive antibody in the absence of donor-specific antibodies.
Design, setting, participants, & measurements: Retrospective observational study of kidney allograft survival among 4058 zero HLA-A-, B-, DR-, and DQB1-mismatched transplant recipients without antibodies to donor kidney antigens encoded by these HLA gene loci.
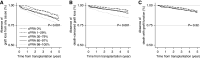
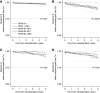
Results: Among 4058 first and repeat transplant recipients, patients with calculated panel reactive antibody (cPRA) 1%-97% were not at higher risk of transplant failure, compared with patients with cPRA of 0% (death censored graft loss: hazard ratio, 1.07; 95% confidence interval, 0.82 to 1.41). Patients with cPRA ≥98% had a higher risk of graft loss from any cause including death (hazard ratio, 1.39; 95% confidence interval, 1.08 to 1.79) and death censored allograft failure (hazard ratio, 1.78; 95% confidence interval, 1.27 to 2.49). In stratified analyses, the higher risk of graft loss among patients with cPRA ≥98% was only observed among repeat, but not first, transplant recipients. In subgroup analysis, there was no association between cPRA and graft loss among living related transplant recipients.
Conclusions: Calculated panel reactive antibody is poorly associated with post-transplant immune reactivity to the allograft in the absence of donor-specific antibody.
Podcast: This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2021_01_25_CJN13640820_final.mp3.
Keywords: acute rejection; antibodies; risk factors; transplant outcomes.
Copyright © 2021 by the American Society of Nephrology.
Figures



References
-
- Tambur AR, Campbell P, Claas FH, Feng S, Gebel HM, Jackson AM, Mannon RB, Reed EF, Tinckam K, Askar M, Chandraker A, Chang PP, Colvin M, Demetris AJ, Diamond JM, Dipchand AI, Fairchild RL, Ford ML, Friedewald J, Gill RG, Glotz D, Goldberg H, Hachem R, Knechtle S, Kobashigawa J, Levine DJ, Levitsky J, Mengel M, Milford E, Newell KA, O’Leary JG, Palmer S, Randhawa P, Smith J, Snyder L, Starling RC, Sweet S, Taner T, Taylor CJ, Woodle S, Zeevi A, Nickerson P: Sensitization in transplantation: Assessment of risk (STAR) 2017 working group meeting report. Am J Transplant 18: 1604–1614, 2018 - PubMed
-
- Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 - PubMed
-
- Gjertson DW, Cecka JM: Determinants of long-term survival of pediatric kidney grafts reported to the United Network for Organ Sharing kidney transplant registry. Pediatr Transplant 5: 5–15, 2001 - PubMed
-
- Opelz G; Collaborative Transplant Study: Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet 365: 1570–1576, 2005 - PubMed
-
- Ibrahim HN, Skeans MA, Li Q, Ishani A, Snyder JJ: Blood transfusions in kidney transplant candidates are common and associated with adverse outcomes. Clin Transplant 25: 653–659, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

